Duchenne muscular dystrophy
2008/9 Schools Wikipedia Selection. Related subjects: Health and medicine
| Duchenne muscular dystrophy Classification and external resources |
|
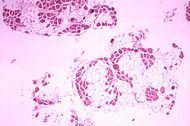
| Histopathology of gastrocnemius muscle from patient who died of pseudohypertrophic muscular dystrophy, Duchenne type. Cross section of muscle shows extensive replacement of muscle fibers by adipose cells. | |
| ICD- 10 | G 71.0 |
| ICD- 9 | 359.1 |
| OMIM | 310200 |
| DiseasesDB | 3985 |
| MedlinePlus | 000705 |
| MeSH | D020388 |
Duchenne muscular dystrophy (DMD) is a severe recessive x-linked form of muscular dystrophy that is characterized by rapid progression of muscle degeneration, eventually leading to loss in ambulation, paralysis, and death. This affliction affects one in 3500 males, making it the most prevalent of muscular dystrophies. In general, males are only afflicted, though females can be carriers. The disorder is caused by a mutation in the gene DMD, located in humans on the X chromosome. The DMD gene codes for the protein dystrophin, an important structural component within muscle tissue. Dystrophin provides structural stability to the dystroglycan complex (DGC), located on the cell membrane.
Symptoms usually appear in male children before age 6 and may be visible in early infancy. Progressive proximal muscle weakness of the legs and pelvis associated with a loss of muscle mass is observed first. Eventually this weakness spreads to the arms, neck, and other areas. Early signs may include pseudohypertrophy (enlargement of calf muscles), low endurance, and difficulties in standing unaided or inability to ascend staircases. As the condition progresses, muscle tissue experiences wasting and is eventually replaced by fat and fibrotic tissue ( fibrosis). By age 10, braces may be required to aide in walking but most patients are wheelchair dependent by age 12. Later symptoms may include abnormal bone development that lead to skeletal deformities, including curvature of the spine. Due to progressive deterioration of muscle, loss of movement occurs eventually leading to paralysis. Intellectual impairment may also be present but does not progressively worsen as the child ages. The average life expectancy for patients afflicted with DMD varies from early teens to age mid 30s. There have been reports of DMD patients surviving past the age of 40 and even 50.
Incidence/prevalence
Duchenne muscular dystrophy is caused by mutations in the DMD gene, which is located on the X chromosome. Due to this, DMD has an incidence of 1 in 4,000 newborn males. Mutations within the DMD gene can either be inherited or occur spontaneously during germline transmission.
Eponym
DMD is named after the French neurologist Guillaume Benjamin Amand Duchenne (1806-1875), who first described the disease in the 1860s.
Pathogenesis
Duchenne muscular dystrophy is caused by a mutation of the dystrophin gene at locus Xp21. Dystrophin is responsible for the connection of muscle fibers to the extracellular matrix through a protein complex containing many subunits. The absence of dystrophin permits excess calcium to penetrate the sarcolemma (cell membrane). In a complex cascading process that involves several pathways and is not clearly understood, increased oxidative stress within the cell damages the sarcolemma, eventually results in the death of the cell. Muscle fibers undergo necrosis and are ultimately replaced with adipose and connective tissue.
Symptoms
The main symptom of Duchenne muscular dystrophy is progressive muscle weakness associated with muscle wasting with the proximal muscles being first affected, especially the pelvis and calf muscles. Muscle weakness also occurs in the arms, neck, and other areas, but not as early as in the lower half of the body. Symptoms usually appear before age 6 and may appear as early as infancy. Generalized weakness and muscle wasting first affecting the muscles of the hips, pelvic area, thighs and shoulders. Calves are often enlarged. The other physical symptoms are:
- Awkward gait (patients tend to walk on their forefeet, because of an increased calve tonus)
- Frequent falls
- Fatigue
- Difficulty with motor skills (running, hopping, jumping)
- Increased Lumbar lordosis, leading to shortening of the hip-flexor muscles. This has an affect on overall posture and gait.
- Muscle contractures of achilles tendon and hamstrings impair functionality because the muscle fibers shorten and fibrosis occurs in connective tissue
- Progressive difficulty walking
- Muscle fibre deformities
- Pseudohypertrophy of tongue and calf muscles. The enlarged muscle tissue is eventually replaced by fat and connective tissue, hence the term pseudohypertrophy.
- Higher risk of behaviour and learning difficulties.
- Eventual loss of ability to walk (usually by the age of 12)
- Skeletal deformities (including scoliosis in some cases)
Signs and tests
Muscle wasting begins in the legs and pelvis, then progresses to the muscles of the shoulders and neck, followed by loss of arm muscles and respiratory muscles. Calf muscle enlargement (pseudohypertrophy) is quite obvious. Cardiomyopathy may occur, but the development of congestive heart failure or arrhythmias (irregular heartbeats) is rare.
- A positive Gower's sign reflects the more severe impairment of the lower extremities muscles. The child helps himself to get up with upper extremities: first by rising to stand on his arms and knees, and then "walking" his hands up his legs to stand upright.
- Affected children usually tire more easily and have less overall strength than their peers.
- Creatine kinase (CPK-MM) levels in the bloodstream are extremely high.
- An electromyography (EMG) shows that weakness is caused by destruction of muscle tissue rather than by damage to nerves.
- Genetic testing can reveal genetic errors in the Xp21 gene.
- A muscle biopsy ( immunohistochemistry or immunoblotting) or genetic test ( blood test) confirms the absence of dystrophin, although improvements in genetic testing often make this unnecessary.
Diagnosis
CPK test
If a physician suspects DMD after examining the boy they will use a CPK ( creatine phosphokinase) test to determine if the muscles are damaged. This test measures the amount of CPK in the blood. In DMD patients CPK leaks out of the muscle cell into the bloodstream, so a high level (50 to 100 times normal) confirms that there is muscle damage. Affected individuals may have a value as high as 15,000 to 35,000iu/l (normal = 60iu/l).
DNA test
The muscle-specific isoform of the dystrophin gene is composed of 79 exons, and DNA testing and analysis can usually identify the specific type of mutation of the exon or exons that are affected. DNA testing confirms the diagnosis in most cases.
Muscle biopsy
If DNA testing fails to find the mutation, a muscle biopsy test may be performed. A small sample of muscle tissue is extracted and a dye is applied that reveals the presence of dystrophin. Complete absence of the protein indicates the condition.
Over the past several years DNA tests have been developed that detect more of the many mutations that cause the condition, and muscle biopsy is not required as often to confirm the presence of Duchenne's.
Prenatal tests
If one or both parents are 'carriers' of a particular condition there is a risk that their unborn child will be affected by that condition. 'Prenatal tests' are carried out during pregnancy, to try to find out if the fetus (unborn child) is affected. The tests are only available for some neuromuscular disorders. Different types of prenatal tests can be carried out after about 10 weeks of pregnancy. Chorion villus sampling (CVS) can be done at 10-12 weeks, and amniocentesis at about 14-16 weeks, while placental biopsy and foetal blood sampling can be done at about 18 weeks. Women and/or couples need to consider carefully which test to have and to discuss this with their genetic counselor. Earlier testing would allow early termination which would probably be less traumatic for the couple, but it carries a slightly higher risk of miscarriage than later testing (about 2%, as opposed to 0.5%).
Treatment
There is no known cure for Duchenne muscular dystrophy, although recent stem-cell research is showing promising vectors that may replace damaged muscle tissue. Treatment is generally aimed at control of symptoms to maximize the quality of life, and include the following.
- Corticosteroids such as prednisone and deflazacort increase energy and strength and defer severity of some symptoms.
- Mild, non-jarring physical activity such as swimming is encouraged. Inactivity (such as bed rest) can worsen the muscle disease.
- Physical therapy is helpful to maintain muscle strength, flexibility, and function.
- Orthopedic appliances (such as braces and wheelchairs) may improve mobility and the ability for self-care. Form-fitting removable leg braces that hold the ankle in place during sleep can defer the onset of contractures.
- Appropriate respiratory support as the disease progresses is important
Prognosis
Duchenne muscular dystrophy eventually affects all voluntary muscles and involves the heart and breathing muscles in later stages. The life expectancy can range from the late teens to the age of 35, However there have been people with duchennes who made it to age 40 and beyond. Recent advancements in medicine are extending the lives of those afflicted. Orthapedic Surgery for scoliosis also extend the life of someone with the illness. All people with the DMD illness are affected differently.
Physical Therapy
Physical therapists are concerned with enabling children to reach their maximum physical potential. Their aim is to:
- minimize the development of contractures and deformity by developing a programme of stretches and exercises where appropriate
- anticipate and minimize other secondary complications of a physical nature
- monitor respiratory function and advise on techniques to assist with breathing exercises and methods of clearing secretions
Mechanical ventilatory/Respiration Assistance
Modern "volume ventilators/respirators," which deliver an adjustable volume (amount) of air to the person with each breath, are valuable in the treatment of people with muscular dystrophy related respiratory problems. Ventilator treatment can begin in the mid to late teens when the respiratory muscles can begin to collapse. However there are people with the disease in their 20's who have no need for a ventillator.
If the vital capacity has dropped below 40 percent of normal, a volume ventilator/respirator may be used during sleeping hours, a time when the person is most likely to be under ventilating ("hypoventilating"). Hypoventilation during sleep is determined by a thorough history of sleep disorder with an oximetry study and a capillary blood gas (See Pulmonary Function Testing). The ventilator may require an endotracheal or tracheotomy tube through which air is directly delivered, however, for some people delivery through a face mask is sufficient.
If the vital capacity continues to decline to less than 30 percent of normal, a volume ventilator/respirator may also be needed during the day for more assistance. The person gradually will increase the amount of time using the ventilator/respirator during the day as needed. A tracheotomy tube may be used in the daytime and during sleep, however, delivery through a face mask may be sufficient. The machine can easily fit on a ventilator tray on the bottom or back of a power wheelchair with an external battery for portability.
Ongoing Research
Promising research is being conducted to find a therapy able to mitigate the detrimental effects of this affliction. There are many avenues currently under intense investigation. One avenue is gene therapy. These therapies include: stem cell replacement, exon-skipping, analog up-regulation, and gene replacement. Another avenue is supportive care which is involved in drug development to stave-off disease progression.
Stem Cell Replacement
Though stem cells isolated from the muscle (satellite cells) have the ability to differentiate into myotubes when injected directly into the muscle of animals, they lack the ability to spread systemically throughout. To effectively deliver a therapeutic dose to an isolated muscle it would require direct injections to that muscle every 2mm. This problem was circumvented by using another multipotent stem cell, termed pericytes, that are located within the blood vessels of skeletal muscle. These cells have the ability to be delivered systemically and uptaken by crossing the vascular barrier. Once past the vasculature, pericytes have the ability to fuse and form myotubes. This means that they can be injected arterially, crossing through arterial walls into muscle, where they can differentiate into pontentially functional muscle. These findings show potential for stem cell therapy of DMD. The pericyte-derived cells would be extracted, grown in culture, and then these cells would be injected into the blood stream where they could navigate into and differentiate into skeletal muscle.
Utrophin Upregulation
Utrophin regulation is of great interest as it serves as the closest endogenous paralog within the human genome. This gene is shorter and located on chromosome 6 in humans. Researchers are currently focusing on understanding the regulation behind its expression within cells. It has been found previously (note Kay Davies) that upregulation of utrophin can partially compensate for muscle cells lacking dystrophin expression.
Supportive Care - Drug Development
Recent research shows Losartan, a currently available drug used for treating hypertension, to be effective in halting the progress of the disease in mice that were genetically engineered to have Duchenne's. Human trials are in planning.
Some parents of children with Duchenne's are noting reductions of symptomatic severity from a regimen of Protandim, a non-prescription nutritional supplement that increases levels of two specific antioxidant enzymes. Other parents report no benefit. Controlled clinical trials have not yet been conducted, and parent observations may have been influenced by confounding factors such as expectation bias, normal developmental progress, and the common practice of implementing additional nutritional supplements and/or corticosteroids concurrent with the Protandim. However, Protandim is promising on a theoretical level, in that it has the potential to modify the inflammatory/cell death cycle. DMD mouse-model trials of the therapy are in progress, and human trials are planned.
Research from a group in France led by L. Ségalat has identified a number of drugs that are currently licensed for other applications as halting or reducing dramatically the advance of muscle degeneration in a worm model of DMD. They are now using mouse models to confirm these findings, which so far are looking very promising, confirming the efficacy of these drugs. However, work in mice seems to be moving slowly. The main classes of drugs they identified were SSRI (i.e. antidepressants such as Prozac) and muscle relaxants, such as those used by athletes after heavy training. There is conflicting evidence from animal models suggesting that doing less exercise slows down the rate of degeneration of the muscle; therefore there is a possibility that both these drugs act somewhat as sedatives, although the reality seems to be that the worms and mice are more active overall, as they have less muscle damage and so can remain active for much longer.
More recently, a group at the Montreal Heart Institute and McGill University reported that a mouse model of Duchenne's muscular dystrophy demonstrated early metabolic alterations that precede overt cardiomyopathy and may represent an early "subclinical" signature of a defective nitric oxide (NO)/ cGMP pathway. Accordingly, they used genetic and pharmacological approaches to test the hypothesis that enhancing cGMP, downstream of NO formation, improves the contractile function, energy metabolism, and sarcolemmal integrity. Treatment with Sildenafil delayed the appearance of symptoms in mouse hearts with Duchenne's and allowed to withstand an acutely increased cardiac workload.
Clinical Trials
More information on the new PTC124 trials, currently nearing the end of Phase II, is available at the MDA.org website. This potential treatment would address from 5 to 15 percent of DMD cases where the dystrophin protein cannot be completed due to an incorrect stop codon in the genetic sequence. The PTC124 treatment skips the improper "stop" instruction, allowing reading through of the remaining sequence and completion of the dystrophin protein assembly process. In recent mouse trials, PTC124 was found to repair damaged muscle tissues. Clinical trials for exon skipping with Morpholino oligos and with 2'-O-methyl phosphorothioate oligos are in progress.
Prevention
Genetic counseling is advised for people with a family history of the disorder. Duchenne muscular dystrophy can be detected with about 95% accuracy by genetic studies performed during pregnancy.
Organizations specific to DMD
In addition to charities devoted to muscular dystrophies in general (such as MDA), these charities are devoted exclusively to DMD:
- United Parent Project Muscular Dystrophy: United Parent Projects MD is an international organisation that has been set up by parents and friends of boys with DMD.
- Parent Project Muscular Dystrophy: Parent Project Muscular Dystrophy’s mission is to improve the treatment, quality of life and long-term outlook for all individuals affected by Duchenne muscular dystrophy (DMD) through research, advocacy, education and compassion.
- Charley's Fund: an organisation whose mission is to fund research for cure or treatment for Duchenne. Charley's Fund invests money in translational research – research that focuses on moving science from the lab into human clinical trials.
- Darius Goes West Foundation: a foundation revolving around the documentary film entitled Darius Goes West (DGW). DGW won over 25 film festival awards in 2007, making it the most honored film on the circuit that year. It features the story of Darius Weems, a fifteen-year-old freshman with DMD, who leaves home with his eleven best friends in an attempt to see his wheelchair customized on MTV's Pimp my ride. Watch Trailer. Every DVD sold raises $17 for DMD research.
- JettFund: Currently, 25 teens are biking across America to raise funds for teens with DMD.
- CureDuchenne: is a non-profit organization that aggressively funds leading edge research for treatments and a cure for Duchenne muscular dystrophy.
- Action Duchenne: exclusively funds research for a cure and promotes campaigns for better medical care for Duchenne and Becker Muscular Dystrophy.
- DREAM Foundation: a Louisville, KY based organization devoted to raising funds and awareness. In addition, the foundation funded (through donation) the construction of five playgrounds in the Louisville area specifically designed for use by children with DMD.