Leprosy
2008/9 Schools Wikipedia Selection. Related subjects: Health and medicine
| Leprosy (Hansen's Disease) Classification and external resources |
||
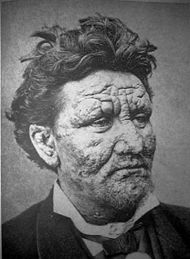
| A 24-year-old man infected with leprosy. | ||
| ICD- 10 | A 30. | |
| ICD- 9 | 030 | |
| OMIM | 246300 | |
| DiseasesDB | 8478 | |
| MedlinePlus | 001347 | |
| eMedicine | med/1281 derm/223 neuro/187 | |
| MeSH | C01.252.410.040.552.386 | |
Leprosy (from the Greek lepid, meaning scales on a fish), or Hansen's disease, is a chronic disease caused by the bacterium Mycobacterium leprae. Leprosy is primarily a granulomatous disease of the peripheral nerves and mucosa of the upper respiratory tract; skin lesions are the primary external symptom. Left untreated, leprosy can be progressive, causing permanent damage to the skin, nerves, limbs and eyes. Contrary to popular conception, leprosy does not actually cause body parts to simply fall off.
Historically, leprosy has affected mankind since at least 600 BC, and was well-recognized in the civilizations of ancient China, Egypt and India. In 1995, the World Health Organization (WHO) estimated that between two and three million people were permanently disabled because of leprosy. Although the forced quarantine or segregation of patients is unnecessary—and can be considered unethical—a few leper colonies still remain around the world, in countries such as India, Japan, Egypt, Argentina and Vietnam. It is now commonly believed that many of the people who were segregated into these communities were presumed to have leprosy, when they actually had syphilis. Leprosy, which is not infectious, would not have spread through a community, where syphilis, which has similar symptoms, is contagious.
The age-old social stigma associated with the advanced form of leprosy lingers in many areas, and remains a major obstacle to self-reporting and early treatment. Effective treatment for leprosy appeared in the late 1930s with the introduction of dapsone and its derivatives. However, leprosy bacilli resistant to dapsone gradually evolved and became widespread, and it was not until the introduction of multidrug therapy (MDT) in the early 1980s that the disease could be diagnosed and treated successfully within the community.
Characteristics
The clinical symptoms of leprosy vary but primarily affect the skin, nerves, and mucous membranes. Patients with this chronic infectious disease are classified as having paucibacillary Hansen's disease (tuberculoid leprosy), multibacillary Hansen's disease (lepromatous leprosy), or borderline leprosy.
Contrary to popular belief, Hansen's bacillus does not cause rotting of the flesh; rather, a long investigation by Paul Brand yielded that insensitivity in the limbs extremities was the reason why unfelt wounds or lesions, however minute, lead to undetected deterioration of the tissues, the lack of pain not triggering an immediate response as in a fully functioning body. Recently, leprosy has also emerged as a problem in HIV patients on antiretroviral drugs.
Classification
There is some confusion over classification because the WHO replaced an older, more complicated classification system with a simpler system that identifies two subtypes of leprosy: paucibacillary and multibacillary. The older system included six categories: Indeterminate Leprosy, Borderline Tuberculoid Leprosy, Midborderline Leprosy, Borderline Lepromatous Leprosy, Lepromatous Leprosy, and Tuberculoid Leprosy.
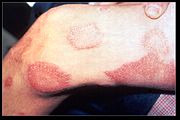
Paucibacillary leprosy encompasses indeterminate, tuberculoid, and borderline tuberculoid leprosy. It is characterized by one or more hypopigmented skin macules and anaesthetic patches, where skin sensations are lost because of damaged peripheral nerves that have been attacked by the human host's immune cells.
Multibacillary leprosy includes midborderline, borderline lepromatous, and lepromatous leprosy. It is associated with symmetric skin lesions, nodules, plaques, thickened dermis, and frequent involvement of the nasal mucosa resulting in nasal congestion and epistaxis (nose bleeds) but typically detectable nerve damage is late.
Borderline leprosy is of intermediate severity and is the most common form. Skin lesions resemble tuberculoid leprosy but are more numerous and irregular; large patches may affect a whole limb, and peripheral nerve involvement with weakness and loss of sensation is common. This type is unstable and may become more like lepromatous leprosy or may undergo a reversal reaction, becoming more like the tuberculoid form.
Cause
Mycobacterium leprae is the causative agent of leprosy. An intracellular, acid-fast bacterium, M. leprae is aerobic, gram-positive, and rod-shaped, and is surrounded by the waxy cell membrane coating characteristic of Mycobacterium species.
Due to extensive loss of genes necessary for independent growth, M. leprae is unculturable in the laboratory, a factor which leads to difficulty in definitively identifying the organism under a strict interpretation of Koch's postulates. The use of non-culture-based techniques such as molecular genetics has allowed for alternative establishment of causation.
Pathophysiology
The exact mechanism of transmission of leprosy is not known: prolonged close contact and transmission by nasal droplet have both been proposed, and, while the latter fits the pattern of disease, both remain unproven. The only other animals besides humans known to contract leprosy are the armadillo, chimpanzee, sooty mangabey, and cynomolgus macaque. The bacterium can also be grown in the laboratory by injection into the footpads of mice. There is evidence that not all people who are infected with M. leprae develop leprosy, and genetic factors have long been thought to play a role, due to the observation of clustering of leprosy around certain families, and the failure to understand why certain individuals develop lepromatous leprosy while others develop other types of leprosy. It is estimated that due to genetic factors, only 5 percent of the population is susceptible to leprosy. This is mostly because the body is naturally immune to the bacteria, and those persons who do become infected are experiencing a severe allergic reaction to the disease. However, the role of genetic factors is not entirely clear in determining this clinical expression. In addition, malnutrition and prolonged exposure to infected persons may play a role in development of the overt disease.
The incubation period for the bacteria can last anywhere from two to ten years.
The most widely held belief is that the disease is transmitted by contact between infected persons and healthy persons. In general, closeness of contact is related to the dose of infection, which in turn is related to the occurrence of disease. Of the various situations that promote close contact, contact within the household is the only one that is easily identified, although the actual incidence among contacts and the relative risk for them appear to vary considerably in different studies. In incidence studies, infection rates for contacts of lepromatous leprosy have varied from 6.2 per 1000 per year in Cebu, Philippines to 55.8 per 1000 per year in a part of Southern India.
Two exit routes of M. leprae from the human body often described are the skin and the nasal mucosa, although their relative importance is not clear. It is true that lepromatous cases show large numbers of organisms deep down in the dermis. However, whether they reach the skin surface in sufficient numbers is doubtful. Although there are reports of acid-fast bacilli being found in the desquamating epithelium (sloughing of superficial layer of skin) of the skin, Weddell et al had reported in 1963 that they could not find any acid-fast bacilli in the epidermis, even after examining a very large number of specimens from patients and contacts. In a recent study, Job et al found fairly large numbers of M. leprae in the superficial keratin layer of the skin of lepromatous leprosy patients, suggesting that the organism could exit along with the sebaceous secretions.
The importance of the nasal mucosa was recognized as early as 1898 by Schäffer, particularly that of the ulcerated mucosa. The quantity of bacilli from nasal mucosal lesions in lepromatous leprosy was demonstrated by Shepard as large, with counts ranging from 10,000 to 10,000,000. Pedley reported that the majority of lepromatous patients showed leprosy bacilli in their nasal secretions as collected through blowing the nose. Davey and Rees indicated that nasal secretions from lepromatous patients could yield as much as 10 million viable organisms per day.
The entry route of M. leprae into the human body is also not definitely known. The two seriously considered are the skin and the upper respiratory tract. While older research dealt with the skin route, recent research has increasingly favored the respiratory route. Rees and McDougall succeeded in the experimental transmission of leprosy through aerosols containing M. leprae in immune-suppressed mice, suggesting a similar possibility in humans. Successful results have also been reported on experiments with nude mice when M. leprae were introduced into the nasal cavity by topical application. In summary, entry through the respiratory route appears the most probable route, although other routes, particularly broken skin, cannot be ruled out. The CDC notes the following assertion about the transmission of the disease: "Although the mode of transmission of Hansen's disease remains uncertain, most investigators think that M. leprae is usually spread from person to person in respiratory droplets."
In leprosy both the reference points for measuring the incubation period and the times of infection and onset of disease are difficult to define; the former because of the lack of adequate immunological tools and the latter because of the disease's slow onset. Even so, several investigators have attempted to measure the incubation period for leprosy. The minimum incubation period reported is as short as a few weeks and this is based on the very occasional occurrence of leprosy among young infants. The maximum incubation period reported is as long as 30 years, or over, as observed among war veterans known to have been exposed for short periods in endemic areas but otherwise living in non-endemic areas. It is generally agreed that the average incubation period is between 3 and 5 years.
Treatment
Until the development of dapsone, rifampicin, and clofazimine in the 1940s, there was no effective cure for leprosy. However, dapsone is only weakly bactericidal against M. leprae and it was considered necessary for patients to take the drug indefinitely. Moreover, when dapsone was used alone, the M. leprae population quickly evolved antibiotic resistance; by the 1960s, the world's only known anti-leprosy drug became virtually useless.
The search for more effective anti-leprosy drugs than dapsone led to the use of clofazimine and rifampicin in the 1960s and 1970s. Later, Indian scientist Shantaram Yawalkar and his colleagues formulated a combined therapy using rifampicin and dapsone, intended to mitigate bacterial resistance. Multidrug therapy (MDT) and combining all three drugs was first recommended by a WHO Expert Committee in 1981. These three anti-leprosy drugs are still used in the standard MDT regimens. None of them are used alone because of the risk of developing resistance.
Because this treatment is quite expensive, it was not quickly adopted in most endemic countries. In 1985 leprosy was still considered a public health problem in 122 countries. The 44th World Health Assembly (WHA), held in Geneva in 1991 passed a resolution to eliminate leprosy as a public health problem by the year 2000 — defined as reducing the global prevalence of the disease to less than 1 case per 100,000. At the Assembly, the World Health Organization (WHO) was given the mandate to develop an elimination strategy by its member states, based on increasing the geographical coverage of MDT and patients’ accessibility to the treatment.
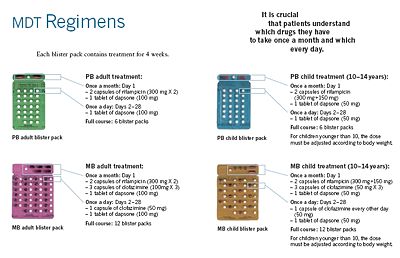
The WHO Study Group's report on the Chemotherapy of Leprosy in 1993 recommended two types of standard MDT regimen be adopted. The first was a 24-month treatment for multibacillary (MB or lepromatous) cases using rifampicin, clofazimine, and dapsone. The second was a six-month treatment for paucibacillary (PB or tuberculoid) cases, using rifampicin and dapsone. At the First International Conference on the Elimination of Leprosy as a Public Health Problem, held in Hanoi the next year, the global strategy was endorsed and funds provided to WHO for the procurement and supply of MDT to all endemic countries.
Between 1995 and 1999, WHO, with the aid of the Nippon Foundation (Chairman Yōhei Sasakawa, World Health Organization Goodwill Ambassador for Leprosy Elimination), supplied all endemic countries with free MDT in blister packs, channelled through Ministries of Health. This free provision was extended in 2000 with a donation by the MDT manufacturer Novartis, which will run until at least the end of 2010. At the national level, non-government organizations (NGOs) affiliated to the national programme will continue to be provided with an appropriate free supply of this WHO supplied MDT by the government.
MDT remains highly effective and patients are no longer infectious after the first monthly dose. It is safe and easy to use under field conditions due to its presentation in calendar blister packs. Relapse rates remain low, and there is no known resistance to the combined drugs. The Seventh WHO Expert Committee on Leprosy, reporting in 1997, concluded that the MB duration of treatment—then standing at 24 months—could safely be shortened to 12 months "without significantly compromising its efficacy."
Persistent obstacles to the elimination of the disease include improving detection, educating patients and the population about its cause, and fighting social taboos about a disease for which patients have historically been considered "unclean" or "cursed by God" as outcasts. Where taboos are strong, patients may be forced to hide their condition (and avoid seeking treatment) to avoid discrimination. The lack of awareness about Hansen's disease can lead people to falsely believe that the disease is highly contagious and incurable.
The ALERT hospital and research facility in Ethiopia provides training to medical personnel from around the world in the treatment of leprosy, as well as treating many local patients. Surgical techniques, such as for the restoration of control of movement of thumbs, have been developed there.
Prevention
A single dose of rifampicin is able to reduce the rate of leprosy in contacts by 57% to 75%.
BCG is able to offer a variable amount of protection against leprosy as well as against tuberculosis.
Epidemiology
Worldwide, two to three million people are estimated to be permanently disabled because of Leprosy. India has the greatest number of cases, with Brazil second and Burma third.
In 1999, the world incidence of Hansen's disease was estimated to be 640,000; in 2000, 738,284 cases were identified. In 1999, 108 cases occurred in the United States. In 2000, the World Health Organization (WHO) listed 91 countries in which Hansen's disease is endemic. India, Myanmar and Nepal contained 70% of cases. In 2002, 763,917 new cases were detected worldwide, and in that year the WHO listed Brazil, Madagascar, Mozambique, Tanzania and Nepal as having 90% of Hansen's disease cases.
According to recent figures from the WHO, new cases detected worldwide have decreased by approximately 107,000 cases (or 21%) from 2003 to 2004. This decreasing trend has been consistent for the past three years. In addition, the global registered prevalence of HD was 286,063 cases; 407,791 new cases were detected during 2004.
Hansen's disease is tracked by the Centers for Disease Control and Prevention (CDC). Its prevalence in the United States is believed to be rising and underreported. Although the number of cases worldwide continues to fall, pockets of high prevalence continue in certain areas such as Brazil, South Asia (India, Nepal), some parts of Africa (Tanzania, Madagascar, Mozambique) and the western Pacific.
Risk groups
At highest risk are those living in endemic areas with poor conditions such as inadequate bedding, contaminated water and insufficient diet, or other diseases (such as HIV) that compromise immune function. Recent research suggests that there is a defect in cell-mediated immunity that causes susceptibility to the disease. Less than ten percent of the world's population is actually capable of acquiring the disease. The region of DNA responsible for this variability is also involved in Parkinson's disease, giving rise to current speculation that the two disorders may be linked in some way at the biochemical level. In addition, men are twice as likely to contract leprosy as women.
According to The Leprosy Mission Canada, most people – about 95% of the population – are naturally immune .
Disease burden
Although annual incidence—the number of new leprosy cases occurring each year—is important as a measure of transmission, it is difficult to measure in leprosy due to its long incubation period, delays in diagnosis after onset of the disease and the lack of laboratory tools to detect leprosy in its very early stages.
Instead, the registered prevalence is used. Registered prevalence is a useful proxy indicator of the disease burden as it reflects the number of active leprosy cases diagnosed with the disease and retrieving treatment with MDT at a given point in time. The prevalence rate is defined as the number of cases registered for MDT treatment among the population in which the cases have occurred, again at a given point in time.
New case detection is another indicator of the disease that is usually reported by countries on an annual basis. It includes cases diagnosed with onset of disease in the year in question (true incidence) and a large proportion of cases with onset in previous years (termed a backlog prevalence of undetected cases). The new case detection rate (NCDR) is defined by the number of newly detected cases, previously untreated, during a year divided by the population in which the cases have occurred.
Endemic countries also report the number of new cases with established disabilities at the time of detection, as an indicator of the backlog prevalence. However, determination of the time of onset of the disease is generally unreliable, is very labour-intensive and is seldom done in recording these statistics.
Global situation
| Table 1: Prevalence at beginning of 2006, and trends in new case detection 2001-2005, excluding Europe | ||||||
| Region | Registered Prevalence (rate/10,000 pop.) |
New Case Detection during the year | ||||
|---|---|---|---|---|---|---|
| Start of 2006 | 2001 | 2002 | 2003 | 2004 | 2005 | |
| Africa | 40,830 (0.56) | 39,612 | 48,248 | 47,006 | 46,918 | 42,814 |
| Americas | 32,904 (0.39) | 42,830 | 39,939 | 52,435 | 52,662 | 41,780 |
| South-East Asia | 133,422 (0.81) | 668,658 | 520,632 | 405,147 | 298,603 | 201,635 |
| Eastern Mediterranean | 4,024 (0.09) | 4,758 | 4,665 | 3,940 | 3,392 | 3,133 |
| Western Pacific | 8,646 (0.05) | 7,404 | 7,154 | 6,190 | 6,216 | 7,137 |
| Totals | NA | 763,262 | 620,638 | 514,718 | 407,791 | 296,499 |
| Table 2: Prevalence and Detection, countries still to reach elimination | ||||||
| Countries | Registered Prevalence (rate/10,000 pop.) |
New Case Detection (rate/100,000 pop.) |
||||
|---|---|---|---|---|---|---|
| Start of 2004 | Start of 2005 | Start of 2006 | During 2003 | During 2004 | During 2005 | |
| 79,908 (4.6) | 30,693 (1.7) | 27,313 (1.5) | 49,206 (28.6) | 49,384 (26.9) | 38,410 (20.6) | |
| 6,810 (3.4) | 4,692 (2.4) | 4,889 (2.5) | 5,907 (29.4) | 4,266 (22.0) | 5,371 (27.1) | |
| 7,549 (3.1) | 4,699 (1.8) | 4,921 (1.8) | 8,046 (32.9) | 6,958 (26.2) | 6,150 (22.7) | |
| 5,420 (1.6) | 4,777 (1.3) | 4,190 (1.1) | 5,279 (15.4) | 5,190 (13.8) | 4,237 (11.1) | |
| Totals | NA | NA | NA | NA | NA | NA |
As reported to WHO by 115 countries and territories in 2006, and published in the Weekly Epidemiological Record the global registered prevalence of leprosy at the beginning of the year was 219,826 cases. New case detection during the previous year (2005 - the last year for which full country information is available) was 296,499. The reason for the annual detection being higher than the prevalence at the end of the year can be explained by the fact that a proportion of new cases complete their treatment within the year and therefore no longer remain on the registers. The global detection of new cases continues to show a sharp decline, falling by 110,000 cases (27%) during 2005 compared with the previous year.
Table 1 shows that global annual detection has been declining since 2001. The African region reported an 8.7% decline in the number of new cases compared with 2004. The comparable figure for the Americas was 20.1%, for South-East Asia 32% and for the Eastern Mediterranean it was 7.6%. The Western Pacific area, however, showed a 14.8% increase during the same period.
Table 2 shows the leprosy situation in the four major countries which have yet to achieve the goal of elimination at the national level. It should be noted that: a) Elimination is defined as a prevalence of less than 1 case per 10,000 population; b) Madagascar reached elimination at the national level in September 2006; c) Nepal detection reported from mid-November 2004 to mid-November 2005; and d) D.R. Congo officially reported to WHO in 2008 that it had reached elimination by the end of 2007, at the national level.
History
Numerous leprosaria, or leper hospitals, sprang up in the Middle Ages; Matthew Paris estimated that in the early thirteenth century there were 19,000 across Europe. The first recorded leprosarium was in Harbledown. (See Leper colony.) These institutions were run along monastic lines and, while lepers were encouraged to live in these monastic-type establishments, this was for their own health as well as quarantine. Indeed, some medieval sources indicate belief that those suffering from leprosy were considered to be going through Purgatory on Earth, and for this reason their suffering was considered more holy than the ordinary person's. More frequently, lepers were seen to exist in a place between life and death: they were still alive, yet many chose or were forced to ritually separate themselves from mundane existence.
Radegund was noted for washing the feet of lepers. Orderic Vitalis writes of a monk, Ralf, who was so overcome by the plight of lepers that he prayed to catch leprosy himself (which he eventually did). The leper would carry a clapper and bell to warn of his approach, and this was as much to attract attention for charity as to warn people that a diseased person was near.
Mycobacterium leprae, the causative agent of leprosy, was discovered by G. H. Armauer Hansen in Norway in 1873, making it the first bacterium to be identified as causing disease in humans. He worked at St. Jørgens Hospital in Bergen, founded early in the fifteenth century. St. Jørgens is today a museum, Lepramuseet, probably the best preserved leprosy hospital in Northern Europe.
Historically, individuals with Hansen's disease have been known as lepers, however, this term is falling into disuse as a result of the diminishing number of leprosy patients and the pejorative connotations of the term. The term most widely accepted among people and agencies working in the field of Hansen's disease is "people affected by Hansen's disease."
Historically, the term Tzaraath from the Hebrew Bible was, erroneously, commonly translated as leprosy, although the symptoms of Tzaraath were not entirely consistent with leprosy and rather referred to a variety of disorders other than Hansen's disease.
In particular, tinea capitis (fungal scalp infection) and related infections on other body parts caused by the dermatophyte fungus Trichophyton violaceum are abundant throughout the Middle East and North Africa today and might also have been common in biblical times. Similarly, the related agent of the disfiguring skin disease favus, Trichophyton schoenleinii, appears to have been common throughout Eurasia and Africa before the advent of modern medicine. Persons with severe favus and similar fungal diseases (and potentially also with severe psoriasis and other diseases not caused by microorganisms) tended to be classed as having leprosy as late as the 17th century in Europe. This is clearly shown in the painting Governors of the Home for Lepers at Haarlem 1667 by Jan de Bray ( Frans Hals Museum, Haarlem, the Netherlands), where a young Dutch man with a vivid scalp infection, almost certainly caused by a fungus, is shown being cared for by three officials of a charitable home intended for leprosy sufferers. The use of the word "leprosy" before the mid-19th century, when microscopic examination of skin for medical diagnosis was first developed, can seldom be correlated reliably with Hansen's disease as we understand it today.
The word "leprosy" derives from the ancient Greek words lepros, a scale, and lepein, to peel. The word came into the English language via Latin and Old French. The first attested English use is in the Ancrene Wisse, a 13th-century manual for nuns ("Moyseses hond..bisemde o þe spitel uuel & þuhte lepruse." The Middle English Dictionary, s.v., "leprous"). A roughly contemporaneous use is attested in the Anglo-Norman Dialogues of Saint Gregory, "Esmondez i sont li lieprous" (Anglo-Norman Dictionary, s.v., "leprus").
Popular culture
- In the film Ben-Hur, Judah's mother and sister suffer from leprosy.
- R.E.M. has a song entitled New Test Leper on their New Adventures in Hi-Fi album.
- The book series The Chronicles of Thomas Covenant, the Unbeliever revolves around the main character's leprosy.
- The Motorcycle Diaries depicts Ernesto Che Guevara, at the age of 24, working at the San Pablo leper colony in Peru: 'Che' was a medical student at the time, specializing in leprosy. He wrote of the poor treatment and living conditions of the patients there. This, and other disparities he witnessed, contributed to his strong belief in the need for public health care.
- "Weird Al" Yankovic has a song entitled " Party at the Leper Colony", in which he plays with expressions such as "footloose", "the cat got her tongue" and "don't give me lip".
- The King of Jerusalem in the 12th century, Baldwin IV, suffered from leprosy. This was portrayed in Kingdom of Heaven, a 2005 film directed by Ridley Scott. The Director's Cut of the film also depicts the king's nephew and heir to the throne, Baldwin V, in the early stages of leprosy.
- The aging Robert the Bruce Sr. in the 1995 film Braveheart, suffers from leprosy.
- Dimmu Borgir has a song entitled Lepers Among Us.
- The famous Vietnamese poet Han Mac Tu suffered and died from leprosy in the 1930s.
- David Bowie describes Ziggy Stardust as a "leper messiah" in the song "Ziggy Stardust".
- The band Metallica has a song called "Leper Messiah" on their Master of Puppets album.
- In The Simpsons episode " Little Big Mom", Lisa tricks Homer and Bart into believing that they have leprosy.
- Death Metal band Death has an album titled Leprosy
- The American TV show House featured a young boy with leprosy in Season 1, Episode 13, "Cursed".
- The book entitled Hawaii by James A. Michener tells one story of a Chinese man, Mun Ki, who discovers that he has leprosy, as termed 'mai Pake'. He is then banished to the isolated leper colony of Kalawao on the island of Moloka'i with other afflicted people.
- In an episode of the American sitcom Seinfeld, Jerry compared the DMV's high concentration of unattractive people to that of a leper colony.
- In the song "One" by the rock band U2, Bono sings "Did you come here for forgiveness? Did you come to raise the dead? Did you come here to play Jesus to the lepers in your head?'
- An episode of the TV series Monk is titled Mr. Monk and the Leper.
- One of the incarnations of the antagonist in Stephen King's It is afflicted with advanced leprosy.
- In the original and remake of the John Carpenter film The Fog, a ship carrying a migrating leper colony is robbed of a fortune to build the fictional Antonio Bay, California, USA.
- In the animated film Princess Mononoke the leader of Irontown, Lady Eboshi, takes in lepers and cares for their wounds and provides shelter for them.
- The band Frightened Rabbit wrote a song called "The Modern Leper".
- The band Opeth wrote a song called "The Leper Affinity", and their song, "Blackwater Park", contains the lines "Lepers coiled 'neath the trees, dying men in bewidered soliloquys".
- Kenshin Himura of the anime Rurouni Kenshin suffered and died of symptoms resembling leprosy in his final days. Though the writers cannot explain his illness, the lesions and the manner of transmission to his wife Kaoru Kamiya resembles that of leprosy.
- The book "The Pearl Diver" written by Jeff Talarigo tells the life story of a 19 year old Japanese pearl diver who discovers that she has leprosy and is sent to a leprosarium on the island of Nagashima where she remains for many years, her name stricken from all records as someone better known as dead.
- In the 1973 film Papillon, Steve McQueen comes across some lepers during one of his escapes from penal colonies. To gain the trust of the lepers McQueen smokes on the leper's cigar, giving the director the opportunity to educate people about non-infectious strains of the bacillus.
- In Monty Python's Life of Brian, a man begging for alms on the street claims to be an ex-leper. When asked about this, he explains he was cured by Jesus. "One minute I'm a leper with a trade, next minute my livelihood's gone."
- Jack London wrote several stories about lepers including "Koolau the Leper" and "The Sheriff of Kona". Leprosy is part of the plot in his book "Michael the Brother of Jerry".
- Sir Arthur Conan Doyle wrote of a sequestered leper in the Sherlock Holmes story "The Adventure of the Blanched Soldier."
Famous People
- Blessed Damien of Moloka'i was a Roman Catholic missionary who became a leper when he spent the rest of his life serving in the leper colony at Moloka'i.
- King Baldwin IV of Jerusalem.
- Possibly Robert I de Brus, King of Scots.