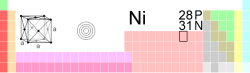
Nickel
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, number | nickel, Ni, 28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 10, 4, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous, metallic and silvery with a gold tinge  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 58.6934 (2) g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 4s2 3d8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 16, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.908 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 7.81 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1728 K (1455 ° C, 2651 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3186 K (2913 ° C, 5275 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 17.48 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 377.5 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 26.07 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face centered cubic 0.3520 nm |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4 , 3, 2, 1 (mildly basic oxide) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.91 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 737.1 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1753.0 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3395 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 135 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 149 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 121 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 163 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 69.3 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 90.9 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 13.4 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) 4900 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 200 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 76 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 180 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 638 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 700 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-02-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nickel (pronounced /ˈnɪkəl/) is a metallic chemical element with the symbol Ni and atomic number 28.
Characteristics
Nickel is a silvery white metal that takes on a high polish. It belongs to the transition metals, and is hard and ductile. It occurs most usually in combination with sulfur and iron in pentlandite, with sulfur in millerite, with arsenic in the mineral nickeline, and with arsenic and sulfur in nickel glance.
It is certain that in common with massive forms of chromium, aluminium and titanium metal that nickel is very slow to react with air, but it is a very reactive element.
Due to its permanence in air and its inertness to oxidation, it is used in coins, for plating iron, brass, etc., for chemical apparatus, and in certain alloys, such as German silver. It is magnetic, and is very frequently accompanied by cobalt, both being found in meteoric iron. It is chiefly valuable for the alloys it forms, especially many superalloys, and particularly stainless steel.
A nickel, which is a 5 cent coin isn't magnetic, because it actually is mostly (75%) copper. The Canadian nickel minted at various periods between 1922-81 was 99.9% nickel, and these are magnetic. Nickel is also a naturally magnetostrictive material, meaning that in the presence of a magnetic field, the material undergoes a small change in length. In the case of Nickel, this change in length is negative (contraction of the material), which is known as negative magnetostriction.
The most common oxidation state of nickel is +2, though 0, +1, +3 and +4 Ni complexes are observed. It is also thought that a +6 oxidation state may exist, however, results are inconclusive.
The unit cell of nickel is a face centered cube with a lattice parameter of 0.356 nm giving a radius of the atom of 0.126 nm.
Nickel-62 is the most stable nuclide of all the existing elements; it is more stable even than Iron-56.
History
The use of nickel is ancient, and can be traced back as far as 3500 BC. Bronzes from what is now Syria had a nickel content of up to 2%. Further, there are Chinese manuscripts suggesting that " white copper" (i.e. baitung) was used in the Orient between 1700 and 1400 BC. However, because the ores of nickel were easily mistaken for ores of silver, any understanding of this metal and its use dates to more contemporary times. Nickel is used today as common household utensils, such as silverware.
Minerals containing nickel (e.g. kupfernickel, meaning copper of the devil ("Nick"), or false copper) were of value for colouring glass green. In 1751, Baron Axel Fredrik Cronstedt was attempting to extract copper from kupfernickel (now called niccolite), and obtained instead a white metal that he called nickel.
In the United States, the term "nickel" or "nick" was originally applied to the copper-nickel Indian cent coin introduced in 1859. Later, the name designated the three-cent coin introduced in 1865, and the following year the five-cent shield nickel appropriated the designation, which has remained ever since. Coins of pure nickel were first used in 1881 in Switzerland.
Occurrence
The bulk of the nickel mined comes from two types of ore deposits. The first are laterites where the principal ore minerals are nickeliferous limonite: (Fe, Ni)O(OH) and garnierite (a hydrous nickel silicate): (Ni, Mg)3Si2O5(OH). The second are magmatic sulfide deposits where the principal ore mineral is pentlandite: (Ni, Fe)9S8.
- see Ore genesis, Category:Nickel minerals
In terms of supply, the Sudbury region of Ontario, Canada, produces about 30 percent of the world's supply of nickel. The Sudbury Basin deposit is theorized to have been created by a massive meteorite impact event early in the geologic history of Earth. Russia contains about 40% of the world's known resources at the massive Norilsk deposit in Siberia. The Russian mining company MMC Norilsk Nickel mines this for the world market, as well as the associated palladium. Other major deposits of nickel are found in New Caledonia, Australia, Cuba, and Indonesia. The deposits in tropical areas are typically laterites which are produced by the intense weathering of ultramafic igneous rocks and the resulting secondary concentration of nickel bearing oxide and silicate minerals. A recent development has been the exploitation of a deposit in western Turkey, especially convenient for European smelters, steelmakers and factories. The one locality in the United States where nickel is commercially mined is Riddle, Oregon, where several square miles of nickel-bearing garnierite surface deposits are located.
Based on geophysical evidence, most of the nickel on Earth is postulated to be concentrated in the Earth's core.
Applications
Nickel is used in many industrial and consumer products, including stainless steel, magnets, coinage, and special alloys. It is also used for plating and as a green tint in glass. Nickel is pre-eminently an alloy metal, and its chief use is in the nickel steels and nickel cast irons, of which there are innumerable varieties. It is also widely used for many other alloys, such as nickel brasses and bronzes, and alloys with copper, chromium, aluminium, lead, cobalt, silver, and gold.
Nickel consumption can be summarized as: nickel steels (60%), nickel-copper alloys and nickel silver (14%), malleable nickel, nickel clad, Inconel and other Superalloys (9%), plating (6%), nickel cast irons (3%), heat and electric resistance alloys, such as Nichrome (3%), nickel brasses and bronzes (2%), others (3%).
In the laboratory, nickel is frequently used as a catalyst for hydrogenation, most often using Raney nickel, a finely divided form of the metal.
Extraction and purification
Nickel can be recovered using extractive metallurgy. Most sulfide ores have traditionally been processed using pyrometallurgical techniques to produce a matte for further refining. Recent advances in hydrometallurgy have resulted in recent nickel processing operations being developed using these processes. Most sulfide deposits have traditionally been processed by concentration through a froth flotation process followed by pyrometallurgical extraction. Recent advances in hydrometallurgical processing of sulfides has led to some recent projects being built around this technology.
Nickel is extracted from its ores by conventional roasting and reduction processes which yield a metal of >75% purity. Final purification of nickel oxides is performed via the Mond process, which upgrades the nickel concentrate to >99.99% purity. This process was patented by L. Mond and was used in South Wales in the 20th century. Nickel is reacted with carbon monoxide at around 50 °C to form volatile nickel carbonyl. Any impurities remain solid. The nickel carbonyl gas is passed into a large chamber at high temperatures in which tens of thousands of nickel spheres are maintained in constant motion. The nickel carbonyl decomposes depositing pure nickel onto the nickel spheres (known as pellets). Alternatively, the nickel carbonyl may be decomposed in a smaller chamber at 230 degrees Celsius to create fine powders. The resultant carbon monoxide is re-circulated through the process. The highly pure nickel produced by this process is known as carbonyl nickel. A second common form of refining involves the leaching of the metal matte followed by the electro-winning of the nickel from solution by plating it onto a cathode. In many stainless steel applications, the nickel can be taken directly in the 75% purity form, depending on the presence of any impurities.
Nickel sulfide ores undergo flotation (differential flotation if Ni/Fe ratio is too low) and then get smelted. Smelting a nickel sulfide flotation concentrate requires a MgO level of <6% otherwise the temperature at which the smelting will be run at will be too high and lead to higher operating costs. After producing the nickel matte, further processing is done via the Sherrit-Gowden process. First copper is removed by adding hydrogen sulfide, leaving a concentrate of only cobalt and nickel. Solvent extration then efficiently separates the cobalt and nickel, with the final nickel concentrate >99%.
In 2005, Russia was the largest producer of nickel with about one-fifth world share closely followed by Canada, Australia and Indonesia, as reported by the British Geological Survey.
Compounds
- Kamacite is a naturally occurring alloy of iron and nickel, usually in the proportion of 90:10 to 95:5 although impurities such as cobalt or carbon may be present. Kamacite occurs in nickel-iron meteorites.
Isotopes
Naturally occurring nickel is composed of 5 stable isotopes; 58Ni, 60Ni, 61Ni, 62Ni and 64Ni with 58Ni being the most abundant (68.077% natural abundance). 18 radioisotopes have been characterised with the most stable being 59Ni with a half-life of 76,000 years, 63Ni with a half-life of 100.1 years, and 56Ni with a half-life of 6.077 days. All of the remaining radioactive isotopes have half-lives that are less than 60 hours and the majority of these have half-lives that are less than 30 seconds. This element also has 1 meta state.
Nickel-56 is produced in large quantities in type Ia supernovae and the shape of the light curve of these supernovae corresponds to the decay via beta radiation of nickel-56 to cobalt-56 and then to iron-56.
Nickel-59 is a long-lived cosmogenic radionuclide with a half-life of 76,000 years. 59Ni has found many applications in isotope geology. 59Ni has been used to date the terrestrial age of meteorites and to determine abundances of extraterrestrial dust in ice and sediment.
Nickel-60 is the daughter product of the extinct radionuclide 60Fe (half-life = 1.5 Myr). Because the extinct radionuclide 60Fe had such a long half-life, its persistence in materials in the solar system at high enough concentrations may have generated observable variations in the isotopic composition of 60Ni. Therefore, the abundance of 60Ni present in extraterrestrial material may provide insight into the origin of the solar system and its early history.
Nickel-62 has the highest binding energy per nucleon of any isotope for any element (8.7946 Mev/nucleon). Isotopes heavier than 62Ni cannot be formed by nuclear fusion without losing energy.
Nickel-48, discovered in 1999, is the most proton-rich heavy element isotope known . With 28 protons and 20 neutrons 48Ni is " doubly magic" (like 208Pb) and therefore unusually stable .
The isotopes of nickel range in atomic weight from 48 u (48-Ni) to 78 u (78-Ni). Nickel-78's half-life was recently measured to be 110 milliseconds and is believed to be an important isotope involved in supernova nucleosynthesis of elements heavier than iron.
Biological role
Nickel plays numerous roles in the biology of microorganisms and plants, though they were not recognized until the 1970s. In fact urease (an enzyme which assists in the hydrolysis of urea) contains nickel. The NiFe- hydrogenases contain nickel in addition to iron-sulfur clusters. Such [NiFe]-hydrogenases characteristically oxidise H2. A nickel-tetrapyrrole coenzyme, F430, is present in the methyl coenzyme M reductase which powers methanogenic archaea. One of the carbon monoxide dehydrogenase enzymes consists of an Fe-Ni-S cluster. Other nickel-containing enzymes include a class of superoxide dismutase and a glyoxalase.
Precautions
Exposure to nickel metal and soluble compounds should not exceed 0.05 mg/cm³ in nickel equivalents per 40-hour work week. Nickel sulfide fume and dust is believed to be carcinogenic, and various other nickel compounds may be as well. Nickel carbonyl, [Ni(CO)4], is an extremely toxic gas. The toxicity of metal carbonyls is a function of both the toxicity of a metal as well as the carbonyl's ability to give off highly toxic carbon monoxide gas, and this one is no exception. It is explosive in air.
Sensitized individuals may show an allergy to nickel affecting their skin, also known as dermatitis. Nickel is an important cause of contact allergy, partly due to its use in jewelry intended for pierced ears. The amount of nickel which is allowed in products which come into contact with human skin is regulated by the European Union. In 2002 researchers found amounts of nickel being emitted by 1 and 2 Euro coins far in excess of those standards. This is believed to be due to a galvanic reaction.
Metal Value
As of April 5, 2007 nickel was trading at 52,300 $US/ mt (52.30 $US/kg, 23.51 $US/lb or 1.47 $US/oz), . Interestingly, the US nickel coin contains 0.04 oz (1.25 g) of nickel, which at this new price is worth 6.5 cents, along with 3.75 grams of copper worth about 3 cents, making the metal value over 9 cents. Since a nickel is worth 5 cents, this made it an attractive target for melting by people wanting to sell the metals at a profit. However, the United States Mint, in anticipation of this practice, implemented new interim rules on December 14, 2006, subject to public comment for 30 days, which criminalize the melting and export of cents and nickels. Violators can be punished with a fine of up to US$10,000 and/or imprisoned for a maximum of five years.
At current use rates, the supply of nickel is predicted to run out in 90 years.