Nitrification
2008/9 Schools Wikipedia Selection. Related subjects: Chemistry; Geology and geophysics
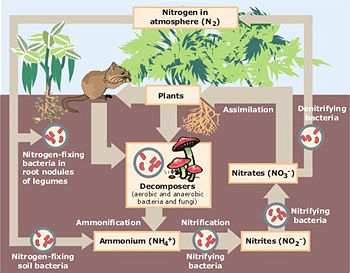
Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil. This process was discovered by the Russian microbiologist, Sergei Winogradsky.
The oxidation of ammonia into nitrite is performed by two groups of organisms, ammonia oxidizing bacteria and ammonia oxidizing archaea. Ammonia oxidizing bacteria can be found among the β- and γ-proteobacteria . In soils the most studied ammonia oxidizing bacteria belong to the genera Nitrosomonas and Nitrosococcus. Although in soils ammonia oxidation occurs by both bacteria and archaea in harsher environments like oceans ammonia oxidation is dominated by archaea. The second step (oxidation of nitrite into nitrate) is (mainly) done by bacteria of the genus Nitrobacter. Both steps are producing energy to be coupled to ATP synthesis. Nitrifying organisms are chemoautotrophs, and use carbon dioxide as their carbon source for growth.
Nitrification also plays an important role in the removal of nitrogen from municipal wastewater. The conventional removal is nitrification, followed by denitrification. The cost of this process resides mainly in aeration (bringing oxygen in the reactor) and the addition of an external carbon source (e.g. methanol) for the denitrification.
In most environments both organisms are found together, yielding nitrate as the final product. It is possible however to design systems in which selectively nitrite is formed (the Sharon process).
Together with ammonification, nitrification forms a mineralization process which refers to the complete decomposition of organic material, with the release of available nitrogen compounds. This replenishes the nitrogen cycle.
Chemistry
Nitrification is a process of nitrogen compound oxidation (effectively, loss of electrons from the nitrogen atom to the oxygen atoms) :
- NH3 + O2 → NO2− + 3H+ + 2e−
- NO2− + H2O → NO3− + 2H+ + 2e−