Radon
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, number | radon, Rn, 86 | |||||||||||||||||||||||||||||||||
| Chemical series | noble gases | |||||||||||||||||||||||||||||||||
| Group, period, block | 18, 6, p | |||||||||||||||||||||||||||||||||
| Appearance | colourless | |||||||||||||||||||||||||||||||||
| Standard atomic weight | (222) g·mol−1 | |||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p6 | |||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 8 | |||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||
| Phase | gas | |||||||||||||||||||||||||||||||||
| Melting point | 202 K (−71.15 ° C, −96 ° F) |
|||||||||||||||||||||||||||||||||
| Boiling point | 211.3 K (−61.85 ° C, −79.1 ° F) |
|||||||||||||||||||||||||||||||||
| Critical point | 377 K, 6.28 MPa | |||||||||||||||||||||||||||||||||
| Heat of fusion | 3.247 kJ·mol−1 | |||||||||||||||||||||||||||||||||
| Heat of vaporization | 18.10 kJ·mol−1 | |||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 20.786 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||
| Crystal structure | cubic face centered | |||||||||||||||||||||||||||||||||
| Oxidation states | 0 | |||||||||||||||||||||||||||||||||
| Electronegativity | 2.2 (Pauling scale) | |||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 1037 kJ·mol−1 | |||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 120 pm | |||||||||||||||||||||||||||||||||
| Covalent radius | 145 pm | |||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||
| Magnetic ordering | non-magnetic | |||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 3.61 m W·m−1·K−1 | |||||||||||||||||||||||||||||||||
| CAS registry number | 10043-92-2 | |||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||
Radon (pronounced /ˈreɪdɒn/) is the chemical element that has the symbol Rn and atomic number 86. Radon is a colorless, naturally occurring, radioactive noble gas that is formed from the decay of radium. It is one of the heaviest substances that are gases under normal conditions and is considered to be a health hazard. The most stable isotope, 222Rn, has a half-life of 3.8 days and is used in radiotherapy. Although due to its radioactivity, it has been less studied by chemists, there are a few known compounds of this unreactive element.
Radon is a significant contaminant that affects indoor air quality worldwide. Radon gas from natural sources can accumulate in buildings and reportedly causes 21,000 lung cancer deaths per year in the United States alone. Radon is the second most frequent cause of lung cancer, after cigarette smoking, and radon-induced lung cancer is thought to be the 6th leading cause of cancer death overall.
History and etymology
Radon is the third discovered radioactive element (after radium and polonium). It was discovered in 1898 by Friedrich Ernst Dorn. In 1900 he reported some experiments in which he noticed that radium compounds emanate a radioactive gas which he named as Radium Emanation (Ra Em). But before that, in 1899, Pierre and Marie Curie observed that the "gas" emitted by radium remained radioactive for a month. That year, Robert B. Owens with Ernest Rutherford noticed variations when trying to measure radiation from thorium oxide. Rutheford noticed that the compounds of thorium continuously emit a radioactive gas which retain the radioactive powers for several minutes and called this gas "emanation" (from Latin "emanare" - to elapse and "emanatio" - expiration), and later Thorium Emanation (Th Em). In 1901 he demonstrated clearly that the emanations are radioactive, but credited the Curies for the discovery of the element. In 1903, similar emanations were observed from actinium by André-Louis Debierne and were called Actinium Emanation (Ac Em).
Several names were suggested for these three gases: exradio, exthorio and exactinio in 1904; radon, thoron and akton in 1918; radeon, thoreon and actineon in 1919, and eventually radon, thoron and actinon in 1920. The likeness of the spectra of these three gases with those of argon, krypton and xenon, and their observed chemical inertia lead Sir William Ramsay to suggest in 1904 that the "emanations" might contain a new element of the noble gas family.
In 1910 Sir William Ramsay and Robert Whytlaw-Gray isolated it, determined its density, and determined that it was the heaviest known gas. They also wrote that "L'expression l'émanation du radium est fort incommode," (the expression of radium emanation is very awkward) and suggested the new name niton (Nt) (from the Latin "nitens" meaning "shining") in order to emphasize the property of gas to cause the phosphorescence of some substances, and in 1912 it was accepted by the International Commission for Atomic Weights. In 1923, the International Committee for Chemical Elements and IUPAC chose for the names: radon (Rn), thoron (Tn), and actinon (An). Later, when isotopes were numbered instead of named, the name of the element took the name of the most stable isotope, radon - while Tn became 220Rn and An 219Rn). As late as the 1960s the element was also referred simply as emanation.
The first synthesized compound of radon was obtained in 1962 and is radon fluoride.
The first major studies of the health concern occurred in the context of uranium mining, first in the Joachimsthal region of Bohemia and then in the Southwestern United States during the early Cold War. Because radon is a product of uranium, uranium mines may have high concentrations of radon and its highly radioactive daughter products. Many uranium miners in the Four Corners region contracted lung cancer and other pathologies as a result of high levels of exposure to radon in the mid-1950s. The increased incidence of lung cancer was particularly pronounced among Native American and Mormon miners, because those groups normally have low rates of lung cancer. Safety standards requiring expensive ventilation were not widely implemented or policed during that period.
The danger of radon exposure in dwellings was discovered in 1984 with the case of Stanley Watras, an employee at the Limerick nuclear power plant in Pennsylvania. Watras set off the radiation alarms on his way into work for two weeks straight while authorities searched for the source of the contamination. They were shocked to find that the source was astonishingly high levels of radon, around 100,000 Bq/m³ (2,700 pCi/L), in his house's basement and it was not related to the nuclear plant. The risks associated with living in his house were estimated to be equivalent to smoking 135 packs of cigarettes every day. Following this event, which was highly publicized, national radon safety standards were set, and radon detection and ventilation became a standard homeowner concern.
Isotopes
Radon has no stable isotopes, but it has 34 radioactive ones that have been studied and they range from an atomic mass of 195 to 228. The most stable isotope is 222Rn, which is a decay product of 226Ra. It has a half-life of 3.823 days and decomposes by alpha particle emission into 218Po. Among the decay daughters of this decay chain is also the highly unstable isotope 218Rn. The naturally occurring 226Ra is a product of the decay chain of 238U. Hereby is this decay series (with half-lives):
- 238U (4.5 x 109 yr) → 234Th (24.1 days) → 234Pa (1.18 min) → 234U (250,000 yr) → 230Th (75,000 yr) → 226Ra (1,600 yr) → 222Rn (3.82 days) → 218Po (3.1 min) → 218At (1.5 s) → 218Rn (35 ms) → 214Pb (26.8 min) → 214Bi (19.7 min) → 214Po (164 µs) → 210Pb (22.3 yr) → 210Bi (5.01 days) → 210Po (138 days) → 206Pb (stable).
There are three other isotopes that have a half life of above 1 hour: 211Rn, 210Rn and 224Rn. The 220Rn isotope is a natural decay product of the most stable thorium isotope (232Th) for which was named “thoron”. It has a half-life of 55.6 seconds and also emits alpha radiation. Similarly, 219Rn is derived from the most stable isotope of actinium (227Ac) — for which it was named “actinon” — and is an alpha emitter with half-life of 3.96 seconds.
Characteristics
An atom of radon is defined as having a nucleus with 86 protons. At standard temperature and pressure, radon forms a monoatomic gas with a density of 9.73 kg/m3, about 8 times the surface density of the Earth's atmosphere, 1.217 kg/m3, and is one of the heaviest gases at room temperature and the heaviest of the noble gases (excluding ununoctium). At standard temperature and pressure radon is a colorless gas, but when it is cooled below its freezing point (202 K ; −71 °C ; −96 °F) it has a brilliant phosphorescence which turns yellow as the temperature is lowered, and becomes orange- red at the temperatures air liquefies (below 93 K ; −180 °C). Upon condensation, radon also glows because of the intense radiation it produces.
Natural radon concentrations in Earth's atmosphere are so low that radon-rich water in contact with the atmosphere will continually lose radon by volatilization. Hence, ground water has a higher concentration of 222Rn than surface water, because the radon is continuously produced by radioactive decay of 226Ra present in rocks. Likewise, the saturated zone of a soil frequently has a higher radon content than the unsaturated zone because of diffusional losses to the atmosphere.
Radon is a health hazard as exposure can cause lung cancer - in fact it is the second major cause of lung cancer after smoking. Radon as a terrestrial source of background radiation is of particular concern because, although on average it is very rare, this intensely radioactive can be found in high concentrations in many areas of the world, where it represents a significant health hazard. Radon-222 has been classified by International Agency for Research on Cancer as being carcinogenic to humans. The contribution to background radiation from radon is so large that it received special attention in the neutrino detection experiments (see Kamioka Observatory).
Radon commercialization is regulated, but it is available in small quantities, at a price of almost $6,000 per mililiter. Because it is also radioactive and is a relatively unreactive chemical element, radon has few uses and is seldom used in academic research.
Chemistry
Radon is a member of the zero-valence elements that are called noble or inert gases. It is inert to most common chemical reactions (such as combustion, for example) because the outer valence shell contains eight electrons. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound. Nevertheless, due to periodic trends, radon has a lower electronegativity than the element above it, xenon, and thus is relatively more reactive.
Because of this price and its radioactivity, experimental chemical research is seldomly done on this element and as a result there are very few reported compounds of radon, all either fluorides or oxides. Radon can be oxidized by a few powerful oxidizing agents. such as F2 thus forming radon fluoride. Among the few other reported compounds of radon are radon oxides.
Occurrence
The average concentration of radon in the atmosphere is about 6×10-20 atoms Rn for each molecule in the air (or about 150 atoms in each mL of air). It can be found in some spring waters and hot springs. The towns of Boulder, Montana, and Misasa; Bad Kreuznach, Germany, as well as the country of Japan boast radium-rich springs which emit radon. Radon emanates naturally from the ground all over the world, particularly in regions with soils containing granite or shale. However, not all granitic regions are prone to high emissions of radon. Radon emitted from the ground has been shown to accumulate in the air if there is a meteorological inversion and little wind. In some caves, increased radon concentration was observed.
Radon is found in some petroleum. Because radon has a similar pressure and temperature curve as propane, and oil refineries separate petrochemicals based on their boiling points, the piping carrying freshly separated propane in oil refineries can become somewhat radioactive due to radon decay particles. Residues from the oil and gas industry often contain radium and its daughters. The sulphate scale from an oil well can be very radium rich, while the water, oil and gas from a well often contains radon. The radon decays to form solid radioisotopes which form coatings on the inside of pipework. In an oil processing plant the area of the plant where propane is processed is often one of the more contaminated areas of the plant as radon has a similar boiling point as propane.
Radon, along with other noble gases krypton and xenon, is also produced during the operation of nuclear power plants. A small fraction of it leaks out of the fuel, through the cladding and into the cooling water, from which it is scavenged. It is then routed to a holding tank where it remains for a large number of half-lives. It is finally purged to the open air through a tall stack which is carefully monitored for radiation level.
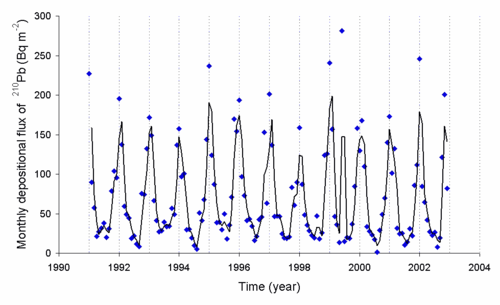
Radon collects over samples of radium 226 at the rate of around 0.001 cm3/day per g of radium. The radon (222Rn) released into the air decays to 210Pb and other radioisotopes, the levels of 210Pb can be measured. The rate of deposition of this radioisotope is dependent on the weather. Here is a graph of the deposition rate observed in Japan. In the early part of the 20th century in the USA, gold which was contaminated with lead-210 entered the jewelry industry. This was from gold seeds which had held radon-222 which had been melted down (after the radon had decayed). The daughters of the radon are still radioactive today.
In 1971, Apollo 15 passed 110 kilometers above the Aristarchus plateau on the Moon, and detected a significant rise in alpha particles thought to be caused by the decay of radon-222. The presence of radon–222 (222Rn) has been inferred later from data obtained from the Lunar Prospector alpha particle spectrometer.
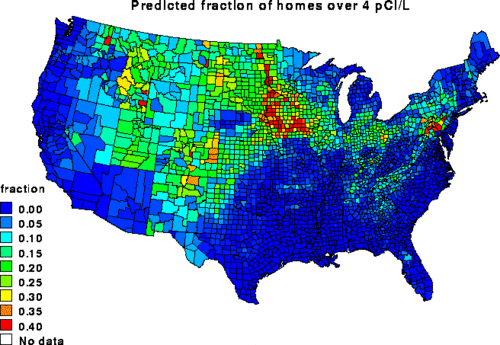
Depending on how houses are built and ventilated, radon may accumulate in basements and dwellings. The highest average radon concentrations in counties in the U.S. are found in Iowa and in the Appalachian Mountain areas in southeastern Pennsylvania. Some of the highest readings ever have been recorded in the Irish town of Mallow, County Cork prompting local fears regarding lung cancer. Iowa has the highest average radon concentrations in the nation due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland. Many cities within the state, such as Iowa City have passed requirements for radon resistant construction in all new homes. A recent study has noted that the counties surrounding Three Mile Island have the highest radon concentrations in the United States and that this may be the cause of the increased lung cancer noted in the region.
The European Union recommends that action should be taken starting from concentrations of 400 Bq/m³ (11 pCi/L) for old houses and 200 Bq/m³ (5 pCi/L) for new ones. After publication of the North American and European Pooling Studies, Health Canada has proposed a new guideline that lowers their action level from 800 to 200 Bq/m³ (22 to 5 pCi/L). The United States Environmental Protection Agency (EPA) strongly recommends action for any house with a concentration higher than 148 Bq/m³ (4 pCi/L), and encourages action starting at 74 Bq/m³ (2 pCi/L). EPA radon risk level tables including comparisons to other risks encountered in life are available in their citizen's guide. Nearly one in 15 homes in the U.S. has a high level of indoor radon according to their statistics. The U.S. Surgeon General and EPA recommend all homes be tested for radon.
Applications
Medical
It has been claimed that exposure to radon gas mitigates auto-immune diseases such as arthritis. As a result, in the late 20th century and early 21st century, some "health mines" were established in Basin, Montana which attracted people seeking relief from health problems such as arthritis through limited exposure to radioactive mine water and radon. The practice was controversial because of the "well-documented ill effects of high-dose radiation on the body."
Radioactive water baths have been applied since 1906 in Jáchymov, Czech Republic, but even before radon discovery they were used in Bad Gastein, Austria. Hot radium-rich spring releasing radon is also used in traditional Japanese onsen in Misasa, Tottori prefecture. Drinking therapy is applied in Bad Brambach, Germany. Inhalation therapy is carried out in Gasteiner-Heilstollen, Austria, in Kowary, Poland and in Boulder, Montana, United States. In the United States and Europe there are a few "radon spas," where people sit for minutes or hours in a high-radon atmosphere in the belief that low doses of radiation will invigorate or energize them.
In addition personal testimonies of arthritis relief and other benefits, there is some (very limited) scientific evidence for this belief, known as hormesis. However, the general scientific community finds it unsubstantiated. There is no known biological mechanism by which such an effect could occur. In addition, it conflicts with the internationally recognized standard that there is no safe threshold for radiation exposure and that exposure should be limited to that "as low as reasonably achievable" (ALARA).
The radon gas which used as a cancer treatment in medicine is obtained from the decay of a radium chloride source. In the past, radium and radon have both been used for X-ray medical radiography, but they have fallen out of use as they are radiotoxic alpha radiation emitters which are expensive and have been replaced with iridium-192 and cobalt-60 since they are far better photon sources.
Scientific
Radon emanation from the soil varies with soil type and with surface uranium content, so outdoor radon concentrations can be used to track air masses to a limited degree. This fact has been put to use by some atmospheric scientists. Because of radon's rapid loss to air and comparatively rapid decay, radon is used in hydrologic research that studies the interaction between ground water and streams. Any significant concentration of radon in a stream is a good indicator that there are local inputs of ground water. Radon is also used in the dating of oil-containing soils because radon has a high affinity of oil-like substances.
Radon soil-concentration has been used in an experimental way to map buried close-subsurface geological faults, because concentrations are generally higher over the faults. Similarly it has found some limited use in geothermal prospecting. Some researchers have even looked at elevated soil-gas radon concentrations, or rapid changes in soil or groundwater radon concentrations, as a predictor for earthquakes. Results have been generally unconvincing but may ultimately prove to have some limited use in specific locations.
Radon is a known pollutant emitted from geothermal power stations, though it disperses rapidly, and no radiological hazard has been demonstrated in various investigations. The trend in geothermal plants is to reinject all emissions by pumping deep underground, and this seems likely to ultimately decrease such radon hazards further.
Testing and mitigation
ASTM E-2121 is a standard for reducing radon in homes as far as practicable below 4 picocuries per liter (pCi/L) in indoor air. ). Radon test kits are commercially available. The kit includes a collector that the user hangs in the lowest livable floor of the house for 2 to 7 days. The user then sends the collector to a laboratory for analysis. The National Environmental Health Association provides a list of radon measurement professionals. Long term kits, taking collections for up to one year, are also available. An open land test kit can test radon emissions from the land before construction begins. The EPA and the National Environmental Health Association have identified 15 types of radon testing. A Lucas cell is one type of device.
Radon levels fluctuate naturally. An initial test might not be an accurate assessment of your home's average radon level. Transient weather can affect short term measurements. Therefore, a high result (over 4 pc/l) justifies repeating the test before undertaking more expensive abatement projects. Measurements between 4 and 10 pc/l warrant a long term radon test. Measurements over 10 pc/l warrant only another short term test so that abatement measures are not unduly delayed. Purchasers of real estate are advised to delay or decline a purchase if the seller has not successfully abated radon to 4 pc/l or less.
The National Environmental Health Association administers a voluntary National Radon Proficiency Program for radon professionals consisting of individuals and companies wanting to take training courses and examinations to demonstrate their competency. A list of mitigation service providers is available. Indoor radon can be mitigated by sealing basement foundations, water drainage, or by sub-slab de-pressurization. In severe cases, mitigation can use air pipes and fans to exhaust sub-slab air to the outside. Indoor ventilation systems are more effective, but exterior ventilation can be cost-effective in some cases. Modern construction that conserves energy by making homes air tight exacerbates the risks of radon exposure if radon is present in the home. Older homes with more porous construction are more likely to vent radon naturally. Ventilation systems can be combined with a heat exchanger to recover energy in the process of exchanging air with the outside. Homes built on a crawl space can benefit from a radon collector installed under a radon barrier (a sheet of plastic that covers the crawl space).
Precautions
Radon is the invisible, radioactive atomic gas that results from radioactive decay of some forms of uranium that may be found in rock formations beneath buildings or in certain building materials themselves. Radon is probably the most pervasive serious hazard for indoor air in the United States and Europe, probably responsible for tens of thousands of lung cancer deaths per annum. There are relatively simple tests for radon gas, but these tests are not commonly done, even in areas of known systematic hazards. Radon is a very heavy gas and thus will tend to accumulate at the floor level. Building materials can actually be a significant source of radon, but very little testing is done for stone, rock or tile products brought into building sites. The half life for radon is 3.8 days, indicating that once the source is removed, the hazard will be greatly reduced within a few weeks. However annually thousands of people go to Radon contaminated mines for purposeful exposure to help with the symptoms of arthritis without any serious health effects.
Radon presents significant risks since it is a colorless and odorless gas and therefore not readily detectable by a human. The radiation decay products ionize genetic material, causing mutations that sometimes turn cancerous. Radon exposure is the second major cause of lung cancer after smoking. Radon gas levels vary by locality and the composition of the underlying soil and rocks. For example, in areas such as Cornwall in the UK (which has granite as substrata), radon gas is a major problem, and buildings have to be force-ventilated with fans to lower radon gas concentrations. The United States Environmental Protection Agency (EPA) estimates that one in 15 homes in the U.S. has radon levels above the recommended guideline of 4 picocuries per liter (pCi/L) (148 Bq/ m³). Iowa has the highest average radon concentration in the United States; studies performed there have demonstrated a 50% increased lung cancer risk with prolonged radon exposure above the EPA's action level of 4 pCi/L.
Radon is a terrestrial source of radiation that is of particular concern because, although on average it is very rare, this intensely radioactive element can be found in high concentrations in many areas of the world, where it represents a significant health hazard. Radon is a decay product of uranium, which is relatively common in the earth's crust, but generally concentrated in ore-bearing rocks scattered around the world. Radon seeps out of these ores into the atmosphere or into ground water, and in these localities it can accumulate within dwellings and expose humans to high concentrations. The widespread construction of well insulated and sealed homes in the northern industrialized world has led to radon becoming the primary source of background radiation in some localities in northern North America and Europe. Some of these areas, including Cornwall and Aberdeenshire in the United Kingdom have high enough natural radiation levels that nuclear licensed sites cannot be built there — the sites would already exceed legal radiation limits before they opened, and the natural topsoil and rock would all have to be disposed of as low-level nuclear waste.
Radiation exposure from radon is indirect. Radon has a short half-life (4 days) and decays into other solid particulate radium-series radioactive nuclides. These radioactive particles are inhaled and remain lodged in the lungs, causing continued exposure. People in affected localities can receive up to 10 mSv per year background radiation. Radon is thus the second leading cause of lung cancer after smoking, and accounts for 15,000 to 22,000 cancer deaths per year in the US alone. The general population is exposed to small amounts of polonium as a radon daughter in indoor air; the isotopes 214Po and 218Po are thought to cause the majority of the estimated 15,000-22,000 lung cancer deaths in the US every year that have been attributed to indoor radon.
The general effects of radon to the human body are caused by its radioactivity and consequent risk of radiation-induced cancer. As an inert gas, radon has a low solubility in body fluids which leads to a uniform distribution of the gas throughout the body. Radon gas and its solid decay products are carcinogens. The greatest health risks come from exposure to the inhaled solid radon gas decay products that are produced during the radioactive decay of radon gas. Two of these decay products, polonium-218 and 214, present a significant radiologic hazard. Once the radioactive decay products are inhaled into the lung, they undergo further radioactive decay, releasing small bursts of energy in the form of alpha particles that can either cause DNA breaks or create free radicals.
It is not known whether radon can cause health effects in other organs besides the lungs. The effects of radon, which is found in food or drinking water, are unknown.
The largest single source of radiation exposure to the general public is naturally-occurring radon gas, which comprises approximately 55% of the annual background dose. The largest natural contributor to public radiation dose is radon, a naturally occurring, radioactive gas found in soil and rock. If the gas is inhaled, some of the radon particles may attach to the inner lining of the lung. These particles continue to decay, emitting alpha particles which can damage cells in the lung tissue.. The death of Marie Curie at age 66 from leukemia was likely caused by prolonged exposure to high doses of ionizing radiation. Curie worked extensively with Radium, which decays into Radon, along with other radioactive materials that emit beta and gamma rays.
In the United Kingdom, residential radon is, after cigarette smoking, the second most frequent cause of lung cancer deaths; 83.9% of deaths are attributed to smoking only, 1.0% to radon only, and 5.5% to a combination of radon and smoking. Radon-induced lung cancer is thought to be the 6th leading cause of cancer death overall. Based on studies carried out by the National Academy of Sciences in the United States, radon is the second most common cause of lung cancer after cigarette smoking, accounting for 15,000 to 22,000 cancer deaths per year in the U.S. The Surgeon General of the United States has reported that over 20,000 Americans die each year of radon-related lung cancer. The United States Environmental Protection Agency (EPA) recommends homes be fixed if an occupant's long-term exposure will average 4 picocuries per liter (pCi/L) (148 Bq m−3) or higher. Beginning with the late 1980s, this lead to activists forming campaigns to raise awareness of radiation resulting from radon.
The most elaborate case-control epidemiologic radon study performed by R. William Field and colleagues demonstrated a 50% increased lung cancer risk with prolonged radon exposure at the EPA's action level of 4 pCi/L. Iowa has the highest average radon concentrations in the nation and a very stable population which added to the strength of the study. Pooled epidemiologic radon studies have also shown an increased lung cancer risk from radon below the EPA's action level of 4 pCi/L.
Radiation from radon has been attribuited to increase of lung cancer among smokers too. This is because the daughters of radon often become attached to smoke and dust particles and are then able to lodge in the lungs. It is unknown whether radon causes other types of cancer, but recent studies suggest a need for further studies to assess the relationship between radon and leukemia. Radon is a common problem encountered during uranium mining as it is a radioactive gas. Inhalation of radon gas caused sharp increases in lung cancers among uranium miners employed in the 1940s and 1950s.
Bernard Cohen has been a staunch opponent to the so called Linear no-threshold model (LNT) which postulates that there is no safe threshold for radiation exposure. His debates in academic periodicals and published correspondence with R. William Field, Brian J. Smith (assistant professor of biostatistics, University of Iowa), Jerry Puskin (from the U.S. Environmental Protection Agency), Sarah Darby, and Sir Richard Doll and others regarding his radon-related ecologic studies are legendary. He offered many rewards ($10,000) if people could provide evidence that the inverse association he found between radon (county averages) and lung cancer (county averages) was due to some factor other than failure of the linear-no threshold theory. Puskin, Smith, Field and others have claimed that his findings are due in part to his inability to control for the inverse association between smoking and radon.