Zinc
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, number | zinc, Zn, 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 12, 4, d | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | bluish pale gray |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 65.409 (4) g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.14 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 6.57 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 692.68 K (419.53 ° C, 787.15 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1180 K (907 ° C, 1665 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.32 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 123.6 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 0.39 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +1(rare) +2 ( amphoteric oxide) |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.65 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 906.4 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1733.3 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3833 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 135 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 142 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 131 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 139 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 59.0 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 116 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 30.2 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) (rolled) 3850 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 108 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 43 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 70 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.25 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 412 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-66-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Zinc (pronounced /ˈzɪŋk/, from German: Zink) is a metallic chemical element with the symbol Zn and atomic number 30. In nonscientific context it is sometimes called spelter.
Notable characteristics
Zinc is a moderately reactive, blue gray metal that tarnishes in moist air and burns in air with a bright bluish-green flame, giving off fumes of zinc oxide. It reacts with acids, alkalis and other non-metals. If not completely pure, zinc reacts with dilute acids to release hydrogen. The one common oxidation state of zinc is +2. From 100 °C to 210 °C (212 °F to 410 °F) zinc metal is malleable and can easily be beaten into various shapes. Above 210 °C (410 °F), the metal becomes brittle and will be pulverized by beating. Zinc is nonmagnetic.
Applications
Zinc is the fourth most common metal in use, trailing only iron, aluminium, and copper in annual production
- Zinc is used to galvanize steel to prevent corrosion
- Zinc is used to Parkerize steel to prevent rust and corrosion
- Zinc is used in alloys such as brass, nickelled silver, typewriter metal, various soldering formulas and German silver
- Zinc is the primary metal used in making American cents since 1982
- Zinc is used in die casting notably in the automobile industry
- Zinc is used as part of the containers of batteries. The most widespread such use is as the anode in alkaline batteries
- Zinc is used as the anode or fuel of the zinc-air battery/fuel cell providing the basis of the theorised zinc economy
- Zinc is used as a sacrificial anode on boats and ships that use cathodic protection to prevent corrosion of metals that are exposed to sea water
- Zinc is used in contemporary pipe organ building as a substitute for the classic lead/tin alloy in pipes sounding the lowest (pedal) tones, as it is tonally almost indistinguishable from lead/tin at those pitches, and has the added advantages of being much more economical and lighter in weight. Even the best organ builders use zinc in this capacity.
- Zinc oxide is used as a white pigment in watercolours or paints, and as an activator in the rubber industry. As an over-the-counter ointment, it is applied as a thin coating on the exposed skin of the face or nose to prevent dehydration of the area of skin. It can protect against sunburn in the summer and windburn in the winter. Applied thinly to a baby's diaper area (perineum) with each diaper change, it can protect against rash. As determined in the Age-Related Eye Disease Study, it is part of an effective treatment for age-related macular degeneration in some cases
- Zinc chloride is used as a deodorant and can also be used as a wood preservative
- Zinc sulfide is used in luminescent pigments such as on the hands of clocks and other items that glow in the dark.
- Zinc methyl (Zn(CH3)2) is used in a number of organic syntheses.
- Zinc stearate is a lubricative plastic additive.
- Lotions made of calamine, a mix of Zn-(hydroxy-)carbonates and silicates, are used to treat skin rash.
- Zinc metal is included in most single tablet over-the-counter daily vitamin and mineral supplements. It is believed to possess anti-oxidant properties, which protect against premature aging of the skin and muscles of the body. In larger amounts, taken as zinc alone in other proprietaries, it is believed by some to speed up the healing process after an injury. Preparations include zinc acetate and zinc gluconate.
- Zinc gluconate glycine and zinc acetate are also used in throat lozenges or tablets to reduce the duration and the severity of cold symptoms.
Popular misconceptions
The highly characteristic metal counters of traditional French bars are often referred to as zinc bars or vaguely zinc, but actually zinc has never been used for this purpose and the counters are really made of an alloy of lead and tin.
History
The name of the metal zinc is unusual and, while vague in origin, was probably first used by Paracelsus, a Swiss-born German chemist who referred to the metal as Zinken or Zinck, in the late 15th century. These words in German apparently mean "tooth-like, pointed or jagged part" and, as zinc metallic crystals are needle-like, the derivation appears plausible. In ancient India the production of zinc metal was very common. Many mine sites of Zawar Mines, near Udaipur, Rajasthan, were active even during 1300–1000 BC. There are references of medicinal uses of zinc in the Charaka Samhita (300 BC). The Rasaratna Samuccaya (800 AD) explains the existence of two types of ores for zinc metal, one of which is ideal for metal extraction while the other is used for medicinal purpose. Zinc alloys have been used for centuries, as brass goods dating to 1400–1000 BC have been found in Israel and zinc objects with 87% zinc have been found in prehistoric Transylvania. Because of the low boiling point and high chemical reactivity of this metal (isolated zinc would tend to go up the chimney rather than be captured), the true nature of this metal was not understood in ancient times.
The manufacture of brass was known to the Ebi by about 30 BC, using a technique where calamine and copper were heated together in a crucible. The zinc oxides in calamine were reduced, and the free zinc metal was trapped by the copper, forming an alloy. The resulting calamine brass was either cast or hammered into shape.
Smelting and extraction of impure forms of zinc was accomplished as early as 1000 AD in India and China. In the West, impure zinc as a remnant in melting ovens was known since Antiquity, but usually discarded as worthless. Strabo mentions it as pseudo-arguros — "mock silver". The Berne zinc tablet is a votive plaque dating to Roman Gaul, probably made from such zinc remnants.
Metallic zinc in the West
The metallurgist Andreas Libavius received in 1597 a quantity of zinc metal in its pure form, which was unknown in the West before then. Libavius identified it as Indian/Malabar lead. Paracelsus (1516) was credited for the name zinc. It was regularly imported to Europe from the orient in the 17th century, but was at times very expensive.
The isolation of metallic zinc in the West may have been achieved independently by several people:
- Traders from the Orient were bringing zinc to England in the early 1700s. It is suggested that they also brought the secret of its smelting, but evidence of this is lacking.
- Dr John Lane is said to have carried out experiments, probably at Landore, prior to his bankruptcy in 1726. Postlewayt's Universal Dictionary, a contemporary source giving technological information in Europe, did not mention zinc before 1751.
- In 1738, William Champion patented in Great Britain a process to extract zinc from calamine in a smelter, using a technology somewhat similar to that used at Zawar zinc mines in Rajasthan. However, there is no evidence that he visited the orient.
- The discovery of pure metallic zinc is also often credited to the German Andreas Marggraf, in 1746, though the whole story is disputed.
Before the discovery of the zinc sulfide flotation technique, calamine was the mineral source of zinc metal.
Biological role
Zinc is an essential element, necessary for sustaining all life. It is estimated that 3,000 of the hundreds of thousands of proteins in the human body contain zinc prosthetic groups, one type of which is the so-called zinc finger. In addition, there are over a dozen types of cells in the human body that secrete zinc ions, and the roles of these secreted zinc signals in medicine and health are now being actively studied. Zinc ions are now considered to be neurotransmitters. Cells in the salivary gland, prostate, immune system and intestine use zinc signalling.
Zinc is also involved in olfaction: the olfactory receptors contain zinc binding sites and a deficiency in zinc causes anosmia.
Zinc is an activator of certain enzymes, such as carbonic anhydrase. Carbonic anhydrase is important in the transport of carbon dioxide in vertebrate blood. It is also required in plants for leaf formation, the synthesis of indole acetic acid (auxin) and anaerobic respiration (alcoholic fermentation).
Zinc deficiency
Zinc deficiency is typically the result of inadequate dietary intake of zinc, disease states that promote zinc losses, or physiological states that require increased zinc. Populations that consume primarily plant based diets that are low in bioavailable zinc often have zinc deficiencies Diseases or conditions that involve intestinal malabsorption promote zinc losses. Fecal losses of zinc caused by diarrhea are one contributing factor , often common in developing countries. Changes in intestinal tract absorbability and permeability due, in part, to viral, protozoal, and bacteria pathogens may also encourage fecal losses of zinc . Physiological states that require increased zinc include periods of growth in infants and children as well as in mothers during pregnancy .
Signs of zinc deficiency include hair loss, skin lesions, diarrhea, and wasting of body tissues. Eyesight, taste, smell and memory are also connected with zinc. A deficiency in zinc can cause malfunctions of these organs and functions. Congenital abnormalities causing zinc deficiency may lead to a disease called Acrodermatitis enteropathica. Conservative estimates suggest that 25% of the world's population is at risk of zinc deficiency.
Zinc supplementation has been shown to reduce diarrhea prevalence and mortality in children <5 years of age.
Zinc deficiency during pregnancy can negatively affect both the mother and fetus. Animal studies indicate that maternal zinc deficiency can upset both the sequencing and efficiency of the birth process. An increased incidence of difficult and prolonged labor, hemorrhage, uterine dystocia and placental abruption has been documented in zinc deficient animals . These effects may be mediated by the defective functioning of estrogen via the estrogen receptor, which contains a zinc finger protein . A review of pregnancy outcomes in women with acrodermatitis enteropathica, reported that out of every seven pregnancies, there was one abortion and two malfunctions, suggesting the human fetus is also susceptible to the teratogenic effects of severe zinc deficiency. However, a review on zinc supplementation trials during pregnancy did not report a significant effect of zinc supplementation on neonatal survival .
Cognitive and motor function may also be impaired in zinc deficient children. Zinc deficiency can interfere with many organ systems especially when it occurs during a time of rapid growth and development when nutritional needs are high, such as during infancy . In animal studies, rats who were deprived of zinc during early fetal development exhibited increased emotionality, poor memory, and abnormal response to stress which interfered with performance in learning situations . Zinc deprivation in monkeys showed that zinc deficient animals were emotionally less mature, and also had cognitive deficits indicated by their difficulty in retaining previously learned problems and in learning new problems . Human observational studies show weaker results. Low maternal zinc status has been associated with less attention during the neonatal period and worse motor functioning . In some studies, supplementation has been associated with motor development in very low birth weight infants and more vigorous and functional activity in infants and toddlers.
It is rarely recognised that lack of zinc can contribute to acne. Leukonychia, white spots on the fingernails, are often seen as an indication of zinc deficiency.
High dose of zinc, 30 mg 1-3 times a day, prevents dysmenorrhea.
Zinc deficiency as a cause of anorexia nervosa
Zinc deficiency causes a decrease in appetite -- which could degenerate in anorexia nervosa (AN). Appetite disorders, in turn, cause malnutrition and, notably, inadequate zinc intake. The use of zinc in the treatment of anorexia nervosa has been advocated since 1979 by Bakan. At least 15 trials showed that zinc improved weight gain in anorexia. A 1994 randomized, double-blind, placebo-controlled trial showed that zinc (14 mg per day) doubled the rate of body mass increase in the treatment of anorexia nervosa (AN). Deficiency of other nutrients such as tyrosine and tryptophan (precursors of the monoamine neurotransmitters norepinephrine and serotonin, respectively), as well as vitamin B1 (thiamine) could contribute to this phenomenon of malnutrition-induced malnutrition.
Zinc toxicity
Even though zinc is an essential requirement for a healthy body, too much zinc can be harmful. Excessive absorption of zinc can also suppress copper and iron absorption. The free zinc ion in solution is highly toxic to plants, invertebrates, and even vertebrate fish. The Free Ion Activity Model (FIAM) is well-established in the literature, and shows that just micromolar amounts of the free ion kills some organisms. A recent example showed 6 micromolar killing 93% of all daphnia in water.
The free zinc ion is also a powerful Lewis acid up to the point of being corrosive. Stomach acid contains hydrochloric acid, in which metallic zinc dissolves readily to give corrosive zinc chloride. Swallowing a post 1982 American one cent piece (97.5% zinc) can cause damage to the stomach lining due to the high solubility of the zinc ion in the acidic stomach. Zinc toxicity, mostly in the form of the ingestion of US pennies minted after 1982, is commonly fatal in dogs where it causes a severe hemolytic anaemia. In pet parrots zinc is highly toxic and poisoning can often be fatal.
There is evidence of induced copper deficiency at low intakes of 100–300 mg Zn/d. The USDA RDA is 15 mg Zn/d. Even lower levels, closer to the RDA, may interfere with the utilization of copper and iron or to adversely affect cholesterol..
Immune system
Zinc salts are effective against pathogens in direct application. Gastroenteritis is strongly attenuated by ingestion of zinc, and this effect could be due to direct antimicrobial action of the zinc ions in the GI tract, or to absorption of the zinc and re-release from immune cells (all granulocytes secrete zinc), or both.
In clinical trials, both zinc gluconate and zinc gluconate glycine (the formulation used in lozenges) have been shown to shorten the duration of symptoms of the common cold. The amount of glycine can vary from two to twenty moles per mole of zinc gluconate.
It should be known that there have been clinical trials that both support the use of zinc for the common cold, and are inconclusive of its effectiveness. All clinical trials have their critics, including the dosage amount used, and the highly subjective format of patient self-reporting the results of their trials.
Abundance
Zinc is the 23rd most abundant element in the Earth's crust. The most heavily mined ores (sphalerite) tend to contain roughly 10% iron as well as 40–50% zinc. Minerals from which zinc is extracted include sphalerite (zinc sulfide), smithsonite (zinc carbonate), hemimorphite (zinc silicate), and franklinite (a zinc spinel).
The earth has been estimated to have 46 years supply of zinc.
Zinc mining and processing
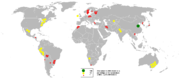
There are zinc mines throughout the world, with the largest producers being China, Australia and Peru. In 2005, China produced almost one-fourth of the global zinc output, reports the British Geological Survey. Mines and refineries in Europe include Umicore in Belgium, Tara, Galmoy and Lisheen in Ireland and Zinkgruvan in Sweden. Zinc metal is produced using extractive metallurgy. Zinc sulfide ( sphalerite) minerals are concentrated using the froth flotation method and then usually roasted using pyrometallurgy to oxidise the zinc sulfide to zinc oxide. The zinc oxide is leached to zinc sulfate (ZnSO4) in several stages of increasingly stronger sulfuric acid (H2SO4). Iron is usually rejected as jarosite or goethite, removing other impurities at the same time. The final purification uses zinc dust to remove copper, cadmium and cobalt in two to three different stages. The metal is then extracted from the purified zinc sulfate solution by electrowinning as cathodic deposits over aluminium sheets. Zinc cathodes can be directly cast or alloyed with aluminium.
Electrolyte zinc sulfate solutions must be very pure for electrowinning to be at all efficient. Impurities can change the decomposition voltage enough to where the electrolysis cell produces largely hydrogen gas rather than zinc metal.
There are two common processes for electrowinning the metal: the low current density process, and the Tainton high current density process. The former uses a 10% sulfuric acid solution as the electolyte, with current density of 270–325 amperes per square meter. The latter uses 22–28% sulfuric acid solution as the electrolyte with a current density of about 1,000 amperes per square meter. The latter gives better purity and has higher production capacity per volume of electrolyte, but has the disadvantage of running hotter and being more corrosive to the vessel in which it is done. In either of the electrolytic processes, each metric ton of zinc production expends about 3900 kW·h (14 MJ) of electric power.
There are also several pyrometallurgical processes that reduce zinc oxide using carbon, then distill the metallic zinc from the resulting mix in an atmosphere of carbon monoxide. These include the Belgian-type horizontal-retort process, the New Jersey Zinc continuous vertical-retort process, and the St. Joseph Lead Company's electrothermal process. The Belgian process requires redistillation to remove impurities of lead, cadmium, iron, copper, and arsenic. The New Jersey process employs a fractionating column, which is absent in the Belgian process, that separates the individual impurities, where they can be sold as byproducts. The St. Joseph Lead Company process heats the zinc oxide/coke mixture by passing an electric current through it rather than by coal or gas fire.
Another pyrometallurgical process is flash smelting. Then zinc oxide is obtained, usually producing zinc of lesser quality than the hydrometallurgical process. Zinc oxide treatment has much fewer applications, but high grade deposits have been successful in producing zinc from zinc oxides and zinc carbonates using hydrometallurgy.
Alloys
The most widely used alloy of zinc is brass, in which copper is alloyed with anywhere from 9% to 45% zinc, depending upon the type of brass, along with much smaller amounts of lead and tin. Alloys of 85–88% zinc, 4–10% copper, and 2–8% aluminium find limited use in certain types of machine bearings. Alloys of primarily zinc with small amounts of copper, aluminium, and magnesium are useful in die casting as well as spin casting. An example of this is zinc aluminium. Similar alloys with the addition of a small amount of lead can be cold-rolled into sheets. An alloy of 96% zinc and 4% aluminium is used to make stamping dies for low production run applications where ferrous metal dies would be too expensive.
Compounds
Zinc oxide is perhaps the best known and most widely used zinc compound, as it makes a good base for white pigments in paint. It also finds industrial use in the rubber industry, and is sold as opaque sunscreen. A variety of other zinc compounds find use industrially, such as zinc chloride (in deodorants), zinc pyrithione (anti- dandruff shampoos), zinc sulfide (in luminescent paints), and zinc methyl or zinc diethyl in the organic laboratory. Roughly one quarter of all zinc output is consumed in the form of zinc compounds.
Isotopes
Naturally occurring zinc is composed of the 5 stable isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn with 64Zn being the most abundant (48.6% natural abundance). Twenty-one radioisotopes have been characterised with the most abundant and stable being 65Zn with a half-life of 244.26 days, and 72Zn with a half-life of 46.5 hours. All of the remaining radioactive isotopes have half-lives that are less than 14 hours and the majority of these have half lives that are less than 1 second. This element also has 4 meta states.
Zinc has been proposed as a " salting" material for nuclear weapons (cobalt is another, better-known salting material). A jacket of isotopically enriched 64Zn, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope Zn-65 with a half-life of 244 days and produce approximately 2.27 MeV of gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several days. Such a weapon is not known to have ever been built, tested, or used.
Precautions
Metallic zinc is not considered to be toxic, but free zinc ions in solution (like copper or iron ions) are highly toxic. There is also a condition called zinc shakes or zinc chills (see metal fume fever) that can be induced by the inhalation of freshly formed zinc oxide formed during the welding of galvanized materials. Excessive intake of zinc can promote deficiency in other dietary minerals.