Insulin
2008/9 Schools Wikipedia Selection. Related subjects: Health and medicine
| Insulin | |
|---|---|
 Insulin crystals |
|
| Genetic data | |
| Locus: | Chr. 11 p15.5 |
| Gene code: | HUGO/ INS |
| Gene type: | Protein codingp |
| Protein Structure/Function | |
| Molecular Weight: | 5808 (Da) |
| Structure: | Solution Structure of Human pro-Insulin Polypeptide |
| Protein type: | insulin family |
| Functions: | glucose regulation |
| Domains: | INS domain |
| Motifs: | SP motif |
| Other | |
| Taxa expressing: | Homo sapiens; homologs: in metazoan taxa from invertebrates to mammals |
| Cell types: | pancreas: beta cells of the Islets of Langerhans |
| Subcellular localization: | extracellular fluids |
| Covalent modifications: | glycation, proteolytic cleavage |
| Pathway(s): | Insulin signaling pathway ( KEGG); Type II diabetes mellitus ( KEGG); Type I diabetes mellitus ( KEGG); Maturity onset diabetes of the young ( KEGG); Regulation of actin cytoskeleton ( KEGG) |
| Receptor/Ligand data | |
| Antagonists: | glucagon, steroids, most stress hormomes |
| Medical/Biotechnological data | |
| Diseases: | familial hyperproinsulinemia, Diabetes mellitus |
| Pharmaceuticals: | insulin ( Humulin Novolin), insulin analogues: insulin lispro ( Humalog), insulin aspart ( NovoLog), insulin detemir ( Levemir), insulin glargine ( Lantus), etc |
| Database Links | |
| Codes: | EntrezGene 3630; Online 'Mendelian Inheritance in Man' (OMIM) 176730; UniProt P01308; RefSeq NM_000207 |
Insulin is a hormone with intensive effects on both metabolism and several other body systems (eg, vascular compliance). When present, it causes most of the body's cells to take up glucose from the blood (including liver, muscle, and fat tissue cells), storing it as glycogen in the liver and muscle, and stops use of fat as an energy source. When insulin is absent (or low), glucose is not taken up by most body cells and the body begins to use fat as an energy source (ie, transfer of lipids from adipose tissue to the liver for mobilization as an energy source). As its level is a central metabolic control mechanism, its status is also used as a control signal to other body systems (such as amino acid uptake by body cells). It has several other anabolic effects throughout the body. When control of insulin levels fail, diabetes mellitus results.
Insulin is used medically to treat some forms of diabetes mellitus. Patients with Type 1 diabetes mellitus depend on external insulin (most commonly injected subcutaneously) for their survival because the hormone is no longer produced internally. Patients with Type 2 diabetes mellitus are insulin resistant, have relatively low insulin production, or both; some patients with Type 2 diabetes may eventually require insulin when other medications fail to control blood glucose levels adequately.
Insulin is a peptide hormone composed of 51 amino acid residues and has a molecular weight of 5808 Da. It is produced in the Islets of Langerhans in the pancreas. The name comes from the Latin insula for "island".
Insulin's structure varies slightly between species of animal. Insulin from animal sources differs somewhat in 'strength' (i.e., in carbohydrate metabolism control effects) in humans because of those variations. Porcine (pig) insulin is especially close to the human version.
Structure
Within vertebrates, the similarity of insulins is extremely close. Bovine insulin differs from human in only three amino acid residues, and porcine insulin in one. Even insulin from some species of fish is similar enough to human to be clinically effective in humans. Insulin in some invertebrates (eg, the c elegans nematode) is quite close to human insulin, has similar effects inside cells, and is produced very similarly. Insulin has been strongly preserved over evolutionary time, suggesting its centrality in animal metabolic control. The C-peptide of proinsulin (discussed later), however, differs much more amongst species; it is also a hormone, but a secondary one.
Mechanism
Insulin is produced in the pancreas, and released when any of several stimuli are detected. These include protein ingestion, and glucose in the blood (from food which produces glucose when digested -- characteristically this is carbohydrate, though not all types produce glucose and so an increase in blood glucose levels). In target cells, they initiate a signal transduction which has the effect of increasing glucose uptake and storage. Finally, insulin is degraded, terminating the response.
Production
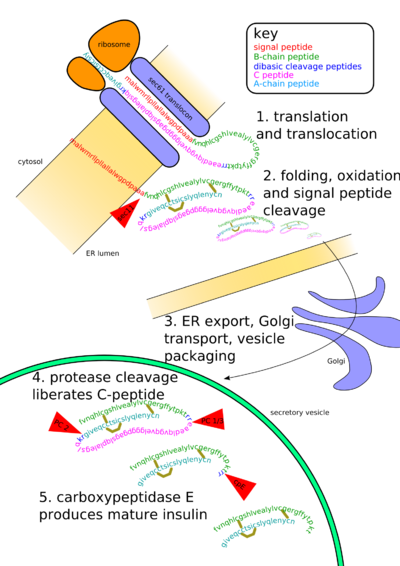
In mammals, insulin is synthesized in the pancreas within the beta cells (β-cells) of the islets of Langerhans. One million to three million islets of Langerhans (pancreatic islets) form the endocrine part of the pancreas, which is primarily an exocrine gland. The endocrine portion only accounts for 2% of the total mass of the pancreas. Within the islets of Langerhans, beta cells constitute 60–80% of all the cells.
In beta cells, insulin is synthesized from the proinsulin precursor molecule by the action of proteolytic enzymes, known as prohormone convertases (PC1 and PC2), as well as the exoprotease carboxypeptidase E. These modifications of proinsulin remove the centre portion of the molecule (ie, C-peptide), from the C- and N- terminal ends of proinsulin. The remaining polypeptides (51 amino acids in total), the B- and A- chains, are bound together by disulfide bonds/disulphide bonds. Confusingly, the primary sequence of proinsulin goes in the order "B-C-A", since B and A chains were identified on the basis of mass, and the C peptide was discovered after the others.
Regulation of production
The endogenous production of insulin is regulated in several steps along the synthesis pathway:
- At transcription from the insulin gene
- In mRNA stability
- At the mRNA translation
- In the posttranslational modifications
Release
Beta cells in the islets of Langerhans release insulin mostly in response to increased blood glucose levels through the following mechanism (see figure to the right):
- Glucose enters the beta cells through the glucose transporter GLUT2
- Glucose goes into the glycolysis and the respiratory cycle where multiple high-energy ATP molecules are produced by oxidation
- Dependent on ATP levels, and hence blood glucose levels, the ATP-controlled potassium channels (K+) close and the cell membrane depolarizes
- On depolarization, voltage controlled calcium channels (Ca2+) open and calcium flows into the cells
- An increased calcium level causes activation of phospholipase C, which cleaves the membrane phospholipid phosphatidyl inositol 4,5-bisphosphate into inositol 1,4,5-triphosphate and diacylglycerol.
- Inositol 1,4,5-triphosphate (IP3) binds to receptor proteins in the membrane of endoplasmic reticulum (ER). This allows the release of Ca2+ from the ER via IP3 gated channels, and further raises the cell concentration of calcium.
- Significantly increased amounts of calcium in the cells causes release of previously synthesised insulin, which has been stored in secretory vesicles
This is the main mechanism for release of insulin and regulation of insulin synthesis. In addition some insulin synthesis and release takes place generally at food intake, not just glucose or carbohydrate intake, and the beta cells are also somewhat influenced by the autonomic nervous system. The signalling mechanisms controlling these linkages are not fully understood.
Other substances known to stimulate insulin release include amino acids from ingested proteins, acetylcholine, released from vagus nerve endings ( parasympathetic nervous system), cholecystokinin[citation needed], released by enteroendocrine cells of intestinal mucosa and glucose-dependent insulinotropic peptide (GIP). Three amino acids (alanine, glycine and arginine) act similarly to glucose by altering the beta cell's membrane potential. Acetylcholine triggers insulin release through phospholipase C, while the last acts through the mechanism of adenylate cyclase.
The sympathetic nervous system (via Alpha2-adrenergic stimulation as demonstrated by the agonists clonidine or methyldopa) inhibit the release of insulin. However, it is worth noting that circulating adrenaline will activate Beta2-Receptors on the Beta cells in the pancreatic Islets to promote insulin release. This is important since muscle cannot benefit from the raised blood sugar resulting from adrenergic stimulation (increased gluconeogenesis and glycogenolysis from the low blood insulin: glucagon state) unless insulin is present to allow for GLUT-4 translocation in the tissue. Therefore, beginning with direct innervation, norepinephrine inhibits insulin release via alpha2-receptors, then subsequently, circulating adrenaline from the adrenal medulla will stimulate beta2-receptors thereby promoting insulin release.
When the glucose level comes down to the usual physiologic value, insulin release from the beta cells slows or stops. If blood glucose levels drop lower than this, especially to dangerously low levels, release of hyperglycemic hormones (most prominently glucagon from Islet of Langerhans' alpha cells) forces release of glucose into the blood from cellular stores, primarily liver cell stores of glycogen. By increasing blood glucose, the hyperglycemic hormones correct life-threatening hypoglycemia. Release of insulin is strongly inhibited by the stress hormone norepinephrine (noradrenaline), which leads to increased blood glucose levels during stress.
Oscillations
Even during digestion, generally one or two hours following a meal, insulin release from pancreas is not continuous, but oscillates with a period of 3–6 minutes, changing from generating a blood insulin concentration more than ~800 p mol/l to less than 100 pmol/l. This is thought to avoid downregulation of insulin receptors in target cells and to assist the liver in extracting insulin from the blood. This oscillation is important to consider when administering insulin-stimulating medication, since it is the oscillating blood concentration of insulin release which should, ideally, be achieved, not a constant high concentration. This may be achieved by delivering insulin rhythmically to the portal vein or by islet cell transplantation to the liver. Future insulin pumps may include this characteristic.
Signal transduction
There are special transporter proteins in cell membranes through which glucose from the blood can enter a cell. These transporters are, indirectly, under blood insulin's control in certain body cell types (e.g., muscle cells). Low levels of circulating insulin, or its absence, will prevent glucose from entering those cells (e.g., in Type 1 diabetes). However, more commonly there is a decrease in the sensitivity of cells to insulin (e.g., the reduced insulin sensitivity characteristic of Type 2 diabetes), resulting in decreased glucose absorption. In either case, there is 'cell starvation', weight loss, sometimes extreme. In a few cases, there is a defect in the release of insulin from the pancreas. Either way, the effect is, characteristically, the same: elevated blood glucose levels.
Activation of insulin receptors leads to internal cellular mechanisms that directly affect glucose uptake by regulating the number and operation of protein molecules in the cell membrane that transport glucose into the cell. The genes that specify the proteins that make up the insulin receptor in cell membranes have been identified and the structure of the interior, cell membrane section, and now, finally after more than a decade, the extra-membrane structure of receptor (Australian researchers announced the work 2Q 2006).
Two types of tissues are most strongly influenced by insulin, as far as the stimulation of glucose uptake is concerned: muscle cells ( myocytes) and fat cells ( adipocytes). The former are important because of their central role in movement, breathing, circulation, etc, and the latter because they accumulate excess food energy against future needs. Together, they account for about two-thirds of all cells in a typical human body.
Effects
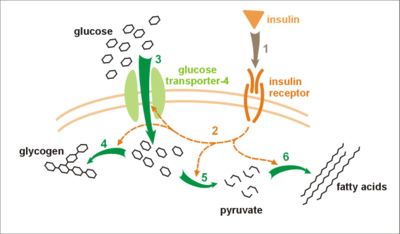
The actions of insulin on the global human metabolism level include:
- Control of cellular intake of certain substances, most prominently glucose in muscle and adipose tissue (about ⅔ of body cells).
- Increase of DNA replication and protein synthesis via control of amino acid uptake.
- Modification of the activity of numerous enzymes.
The actions of insulin on cells include:
- Increased glycogen synthesis – insulin forces storage of glucose in liver (and muscle) cells in the form of glycogen; lowered levels of insulin cause liver cells to convert glycogen to glucose and excrete it into the blood. This is the clinical action of insulin which is directly useful in reducing high blood glucose levels as in diabetes.
- Increased fatty acid synthesis – insulin forces fat cells to take in blood lipids which are converted to triglycerides; lack of insulin causes the reverse.
- Increased esterification of fatty acids – forces adipose tissue to make fats (i.e., triglycerides) from fatty acid esters; lack of insulin causes the reverse.
- Decreased proteolysis – decreasing the breakdown of protein.
- Decreased lipolysis – forces reduction in conversion of fat cell lipid stores into blood fatty acids; lack of insulin causes the reverse.
- Decreased gluconeogenesis – decreases production of glucose from non-sugar substrates, primarily in the liver (remember, the vast majority of endogenous insulin arriving at the liver never leaves the liver) ; lack of insulin causes glucose production from assorted substrates in the liver and elsewhere.
- Increased amino acid uptake – forces cells to absorb circulating amino acids; lack of insulin inhibits absorption.
- Increased potassium uptake – forces cells to absorb serum potassium; lack of insulin inhibits absorption. Thus lowers potassium levels in blood.
- Arterial muscle tone – forces arterial wall muscle to relax, increasing blood flow, especially in micro arteries; lack of insulin reduces flow by allowing these muscles to contract.
Degradation
Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment or it may be degraded by the cell. Degradation normally involves endocytosis of the insulin-receptor complex followed by the action of insulin degrading enzyme. Most insulin molecules are degraded by liver cells. It has been estimated that a typical insulin molecule that is produced endogenously by the pancreatic beta cells is finally degraded about 71 minutes after its initial release into circulation.
Hypoglycemia
Although other cells can use other fuels for a while (most prominently fatty acids), neurons depend on glucose as a source of energy in the non-starving human. They do not require insulin to absorb glucose, unlike muscle and adipose tissue, and they have very small internal stores of glycogen. Glycogen stored in liver cells (unlike glycogen stored in muscle cells) can be converted to glucose, and released into the blood, when glucose from digestion is low or absent, and the glycerol backbone in triglycerides can also be used to produce blood glucose.
Sufficient lack of glucose and scarcity of these sources of glucose can dramatically make itself manifest in the impaired functioning of the central nervous system; dizziness, speech problems, and even loss of consciousness, can occur. Low glucose is known as hypoglycemia or, in cases producing unconsciousness, "hypoglycemic coma" (sometimes termed "insulin shock" from the most common causative agent). Endogenous causes of insulin excess (such as an insulinoma) are very rare, and the overwhelming majority of insulin-excess induced hypoglycemia cases are iatrogenic and usually accidental. There have been a few reported cases of murder, attempted murder, or suicide using insulin overdoses, but most insulin shocks appear to be due to errors in dosage of insulin (e.g., 20 units of insulin instead of 2) or other unanticipated factors (didn't eat as much as anticipated, or exercised more than expected, or unpredicted kinetics of the subcutaneously injected insulin itself).
Possible causes of hypoglycemia include:
- External insulin (usually injected subcutaneously).
- Oral hypoglycemic agents (e.g., any of the sulfonylureas, or similar drugs, which increase insulin release from beta cells in response to a particular blood glucose level).
- Ingestion of low-carbohydrate sugar substitutes (animal studies show these can trigger insulin release (albeit in much smaller quantities than sugar) according to a report in Discover magazine, August 2004, p18). Note that this can never be a cause of hypoglycemia in type 1 diabetes mellitus, as endogenous insulin production no longer exists.
Diseases and syndromes
There are several conditions in which insulin disturbance is pathologic:
- Diabetes mellitus – general term referring to all states characterized by hyperglycemia.
- Type 1 – autoimmune-mediated destruction of insulin producing beta cells in the pancreas resulting in absolute insulin deficiency.
- Type 2 – multifactoral syndrome with combined influence of genetic susceptibility and influence of environmental factors, the best known being obesity, age, and physical inactivity, resulting in insulin resistance in cells requiring insulin for glucose absorption. This form of diabetes is strongly inherited.
- Other types of impaired glucose tolerance (see the diabetes article).
- Insulinoma - a tumor of pancreatic beta cells producing excess of insulin or reactive hypoglycemia.
- Metabolic syndrome – a poorly understood condition first called Syndrome X by Gerald Reaven, Reaven's Syndrome after Reaven, CHAOS in Australia (from the signs which seem to travel together), and sometimes prediabetes. It is currently not clear whether these signs have a single, treatable cause, or are the result of body changes leading to type 2 diabetes. It is characterized by elevated blood pressure, dyslipidemia (disturbances in blood cholesterol forms and other blood lipids), and increased waist circumference (at least in populations in much of the developed world). The basic underlying cause may be the insulin resistance of type 2 diabetes which is a diminished capacity for insulin response in some tissues (e.g., muscle, fat) to respond to insulin. Commonly, morbidities such as essential hypertension, obesity, Type 2 diabetes, and cardiovascular disease (CVD) develop.
- Polycystic ovary syndrome – a complex syndrome in women in the reproductive years where there is anovulation and androgen excess commonly displayed as hirsutism. In many cases of PCOS insulin resistance is present.
As a medication
Principles
Insulin is required for all animal life (excluding certain insects). Its mechanism of action is almost identical in nematode worms (e.g. C. elegans), fish, and mammals, and it is a protein that has been highly conserved across evolutionary time. Insulin must be administered to patients who experience such a deprivation. Clinically, this condition is called diabetes mellitus type 1.
The initial sources of insulin for clinical use in humans were cow, horse, pig or fish pancreases. Insulin from these sources is effective in humans as it is nearly identical to human insulin (three amino acid difference in bovine insulin, one amino acid difference in porcine). Differences in suitability of beef, pork, or fish derived insulin for individual patients have historically been due to lower preparation purity resulting in allergic reactions to the presence of non-insulin substances. Though purity has improved steadily since the 1920s ultimately reaching purity of 99% by the mid-1970s thanks to high-pressure liquid chromatography (HPLC) methods, but minor allergic reactions still occur occasionally, although the same types of allergic reactions have also been known to occur in response to synthetic "human" insulin varieties. Insulin production from animal pancreases was widespread for decades, but very few patients today rely on insulin from animal sources, largely because few pharmaceutical companies sell it anymore.
Synthetic "human" insulin is now manufactured for widespread clinical use using genetic engineering techniques using recombinant DNA technology, which the manufacturers claim reduces the presence of many impurities. Eli Lilly marketed the first such insulin, Humulin, in 1982. Humulin was the first medication produced using modern genetic engineering techniques in which actual human DNA is inserted into a host cell (E. coli in this case). The host cells are then allowed to grow and reproduce normally, and due to the inserted human DNA, they produce a synthetic version of human insulin. However, the clinical preparations prepared from such insulins differ from endogenous human insulin in several important respects; an example is the absence of C-peptide which has in recent years been shown to have systemic effects itself.
Genentech developed the technique Lilly used to produce Humulin, although the company never commercially marketed the product themselves. Novo Nordisk has also developed a genetically engineered insulin independently. According to a survey that the International Diabetes Federation conducted in 2002 on the access to and availability of insulin in its member countries, approximately 70% of the insulin that is currently sold in the world is recombinant, biosynthetic 'human' insulin. A majority of insulin used clinically today is produced this way, although the clinical evidence has provided conflicting evidence on whether these insulins are any less likely to produce an allergic reaction. Also, the International Diabetes Federation's position statement is very clear in stating that "there is NO overwhelming evidence to prefer one species of insulin over another" and "[modern, highly-purified] animal insulins remain a perfectly acceptable alternative."
Since January 2006, all insulins distributed in the U.S. and some other countries are synthetic "human" insulins or their analogs. A special FDA importation process is required to obtain bovine or porcine derived insulin for use in the U.S., although there may be some remaining stocks of porcine insulin made by Lilly in 2005 or earlier.
There are several problems with insulin as a clinical treatment for diabetes:
- Mode of administration.
- Selecting the 'right' dose and timing.
- Selecting an appropriate insulin preparation (typically on 'speed of onset and duration of action' grounds).
- Adjusting dosage and timing to fit food intake timing, amounts, and types.
- Adjusting dosage and timing to fit exercise undertaken.
- Adjusting dosage, type, and timing to fit other conditions, for instance the increased stress of illness.
- Variability in absorption into the bloodstream via subcutaneous delivery
- The dosage is non-physiological in that a subcutaneous bolus dose of insulin alone is administered instead of combination of insulin and C-peptide being released gradually and directly into the portal vein.
- It is simply a nuisance for patients to inject whenever they eat carbohydrate or have a high blood glucose reading.
- It is dangerous in case of mistake (most especially 'too much' insulin).
Types
Medical preparations of insulin (from the major suppliers – Eli Lilly, Novo Nordisk, and Sanofi Aventis – or from any other) are never just 'insulin in water'. Clinical insulins are specially prepared mixtures of insulin plus other substances. These delay absorption of the insulin, adjust the pH of the solution to reduce reactions at the injection site, and so on.
Slight variations of the human insulin molecule are called insulin analogs, so named because they are not technically insulin, rather they are analogs which retain the hormone's glucose management functionality. They have absorption and activity characteristics not currently possible with subcutaneously injected insulin proper. They are either absorbed rapidly in an attempt to mimic real beta cell insulin (as with Lilly's lispro, Novo Nordisk's aspart and Sanofi Aventis' glulisine), or steadily absorbed after injection instead of having a 'peak' followed by a more or less rapid decline in insulin action (as with Novo Nordisk's version Insulin detemir and Sanofi Aventis's Insulin glargine), all while retaining insulin's glucose-lowering action in the human body. However, a number of meta-analyses, including those done by the Cochrane Collaboration in the United Kingdom in 2002, Germany's Institute for Quality and Cost Effectiveness in the Health Care Sector [IQWiG] released in 2007, and the Canadian Agency for Drugs and Technology in Health (CADTH), also released in 2007 have proven unequivocally that any claims of superiority for insulin analogs over regular insulin are unsupported by clinical evidence.
Choosing insulin type and dosage/timing should be done by an experienced medical professional working with the diabetic patient.
The commonly used types of insulin are:
- Rapid-acting, are presently insulin analogs, such as the insulin analog aspart or lispro – begins to work within 5 to 15 minutes and is active for 3 to 4 hours. Newer varieties are in now in Phase II clinical trials which are designed to work rapidly, but retain the same genetic structure as regular human insulin.
- Short-acting, such as regular insulin – starts working within 30 minutes and is active about 5 to 8 hours.
- Intermediate-acting, such as NPH, or lente insulin – starts working in 1 to 3 hours and is active 16 to 24 hours.
- Long-acting, such as ultralente insulin – starts working in 4 to 6 hours, and is active 24 to 28 hours.
- Insulin glargine and Insulin detemir – both insulin analogs which start working within 1 to 2 hours and continue to be active, without major peaks or dips, for about 24 hours, although this varies in many individuals.
- A mixture of NPH and regular insulin – starts working in 30 minutes and is active 16 to 24 hours. There are several variations with different proportions of the mixed insulins.
Yeast-based
In late 2003, Wockhardt commenced manufacture of a yeast-based insulin costing $3.25 in India claiming it eliminates the risk of contracting diseases such as BSE and CJD associated with insulin derived from pigs and cattle. However, the company continues to manufacture insulin derived from pigs in the United Kingdom.
Modes of administration
Unlike many medicines, insulin cannot be taken orally. Like nearly all other proteins introduced into the gastrointestinal tract, it is reduced to fragments (even single amino acid components), whereupon all 'insulin activity' is lost.
Subcutaneous
Insulin is usually taken as subcutaneous injections by single-use syringes with needles, an insulin pump, or by repeated-use insulin pens with needles.
Administration schedules attempt to mimic the physiologic secretion of insulin by the pancreas. Hence, both a long-acting insulin and a short-acting insulin are typically used.
Insulin pump
Insulin pumps are a reasonable solution for some. Advantages to the patient are better control over background or 'basal' insulin dosage, bolus doses calculated to fractions of a unit, and calculators in the pump that may help with determining 'bolus' infusion doages. The limitations are cost, the potential for hypoglycemic and hyperglycemic episodes, catheter problems, and no "closed loop" means of controlling insulin delivery based on current blood glucose levels.
Insulin pumps may be like 'electrical injectors' attached to a temporarily implanted catheter or cannula. Some who cannot achieve adequate glucose control by conventional (or jet) injection are able to do so with the appropriate pump.
As with injections, if too much insulin is delivered or the patient eats less than he or she dosed for, there will be hypoglycemia. On the other hand, if too little insulin is delivered, there will be hyperglycemia. Both can be life-threatening. In addition, indwelling catheters pose the risk of infection and ulceration, and some patients may also develop lipodystrophy due to the infusion sets. These risks can often be minimized by keeping infusion sites clean. Insulin pumps require care and effort to use correctly. However, some patients with diabetes are capable of keeping their glucose in reasonable control only with an insulin pump.
Inhalation
In 2006 the U.S. Food and Drug Administration approved the use of Exubera, the first inhalable insulin. It has been withdrawn from the market by its maker as of 3Q 2007, due to lack of acceptance.
Inhaled insulin has similar efficacy to injected insulin, both in terms of controlling glucose levels and blood half-life. Currently, inhaled insulin is short acting and is typically taken before meals; an injection of long-acting insulin at night is often still required. When patients were switched from injected to inhaled insulin, no significant difference was found in HbA1c levels over three months. Accurate dosing is still a problem, although patients showed no significant weight gain or pulmonary function decline over the length of the trial, when compared to the baseline. Following its commercial launch in 2005 in the UK, it was not (as of July 2006) recommended by National Institute for Health and Clinical Excellence for routine use, except in cases where there is "proven injection phobia diagnosed by a psychiatrist or psychologist".
In January 2008, the world's largest insulin manufacturer, Novo Nordisk A/S, also announced that the company was discontinuing all further development of the company's own version of inhalable insulin, known as the AERx iDMS inhaled insulin system. Similarly, Eli Lilly and Company ended its efforts to develop its Air inhaled insulin in March 2008. MannKind Corp. (whose majority owner, Alfred E. Mann, remained unusually bullish on the concept in spite of Pfizer's costly failure
Transdermal
There are several methods for transdermal delivery of insulin. Pulsatile insulin uses microjets to pulse insulin into the patient, mimicking the physiological secretions of insulin by the pancreas. Jet injection had different insulin delivery peaks and durations as compared to needle injection. Some diabetics find control possible with jet injectors, but not with hypodermic injection.
Both electricity using iontophoresis and ultrasound have been found to make the skin temporarily porous. The insulin administration aspect remains experimental, but the blood glucose test aspect of 'wrist appliances' is commercially available.
Researchers have produced a watch-like device that tests for blood glucose levels through the skin and administers corrective doses of insulin through pores in the skin.
Intranasal insulin
Intranasal insulin is being investigated.
Oral insulin
The basic appeal of oral hypoglycemic agents is that most people would prefer a pill to an injection. However, insulin is a protein, which are digested in the stomach and gut and in order to be effective at controlling blood sugar, can not be taken orally.
The potential market for an oral form of insulin is assumed to be enormous, thus many laboratories have attempted to devise ways of moving enough intact insulin from the gut to the portal vein to have a measurable effect on blood sugar. As of 2004, no products appear to be successful enough yet to bring to market.
A Connecticut-based biopharmaceutical company called Biodel, Inc. is developing what it calls VIAtab, an oral formulation of insulin designed to be administered sublingually. This therapy is a tablet that dissolves in minutes when placed under the tongue. In a Phase I study, VIAtab delivered insulin to the blood stream quickly and resembled the first-phase insulin release spike found in healthy individuals. The company claims that an oral insulin therapy would be more convenient than currently available injectable or inhalable therapies, and they expect that convenience to result in increased insulin usage among the currently underserved early-stage patients with Type 2 diabetes, thus helping to create better long-term outcomes for that patient population.
An Israeli pharmaceutical company, Oramed Pharmaceuticals, is currently conducting Phase 2A studies on an oral insulin pill.
Australian biopharmaceutical company, Apollo Life Sciences, plans to enter the Phase I trial of its oral insulin tablet in mid-2008.
Pancreatic transplantation
Another improvement would be a transplantation of the pancreas or beta cell to avoid periodic insulin administration. This would result in a self-regulating insulin source. Transplantation of an entire pancreas (as an individual organ) is difficult and relatively uncommon. It is often performed in conjunction with liver or kidney transplant, although it can be done by itself. It is also possible to do a transplantation of only the pancreatic beta cells. However, islet transplants had been highly experimental (for which read 'prone to failure') for many years, but some researchers in Alberta, Canada, have developed techniques with a high initial success rate (about 90% in one group). Nearly half of those who got an islet cell transplant were insulin-free one year after the operation; by the end of the second year that number drops to about one in seven. Beta cell transplant may become practical in the near future. Additionally, some researchers have explored the possibility of transplanting genetically engineered non-beta cells to secrete insulin. Clinically testable results are far from realization at this time. Several other non-transplant methods of automatic insulin delivery are being developed in research labs, but none is close to clinical approval.
Artificial pancreas
Dosage and timing
The central problem for those requiring external insulin is picking the right dose of insulin and the right timing.
Physiological regulation of blood glucose, as in the non-diabetic, would be best. Increased blood glucose levels after a meal is a stimulus for prompt release of insulin from the pancreas. The increased insulin level causes glucose absorption and storage in cells, reducing glycogen to glucose conversion, reducing blood glucose levels, and so reducing insulin release. The result is that the blood glucose level rises somewhat after eating, and within an hour or so, returns to the normal 'fasting' level. Even the best diabetic treatment with synthetic human insulin or even insulin analogs, however administered, falls far short of normal glucose control in the non-diabetic.
Complicating matters is that the composition of the food eaten (see glycemic index) affects intestinal absorption rates. Glucose from some foods is absorbed more (or less) rapidly than the same amount of glucose in other foods. Fats and proteins cause delays in absorption of glucose from carbohydrate eaten at the same time. As well, exercise reduces the need for insulin even when all other factors remain the same, since working muscle has some ability to take up glucose without the help of insulin.
It is, in principle, impossible to know for certain how much insulin (and which type) is needed to 'cover' a particular meal to achieve a reasonable blood glucose level within an hour or two after eating. Non-diabetics' beta cells routinely and automatically manage this by continual glucose level monitoring and insulin release. All such decisions by a diabetic must be based on experience and training (i.e., at the direction of a physician, PA, or in some places a specialist diabetic educator) and, further, specifically based on the individual experience of the patient. But it is not straightforward and should never be done by habit or routine. With some care however, it can be done reasonably well in clinical practice.
For example, some patients with diabetes require more insulin after drinking skim milk than they do after taking an equivalent amount of fat, protein, carbohydrate, and fluid in some other form. Their particular reaction to skimmed milk is different from other people with diabetes, but the same amount of whole milk is likely to cause a still different reaction even in that person. Whole milk contains considerable fat while skimmed milk has much less. It is a continual balancing act for all people with diabetes, especially for those taking insulin.
Patients with insulin-dependent diabetes require some base level of insulin (basal insulin), as well as short-acting insulin to cover meals (bolus insulin). Maintaining the basal rate and the bolus rate is a continuous balancing act that people with insulin-dependent diabetes must manage each day. This is normally achieved through regular blood tests, although continuous blood sugar testing equipment (Continuous Glucose Monitors or CGMs) are now becoming available.
Strategies
A long-acting insulin is used to approximate the basal secretion of insulin by the pancreas. NPH/isophane, lente, ultralente, glargine, and detemir may be used for this purpose. The advantage of NPH is its low cost and the fact that you can mix it with short-acting forms of insulin, thereby minimizing the number of injections that must be administered. The disadvantage is that the activity of NPH is less steady and will peak 4–6 hours after administration, and this peak has the potential of causing hypoglycemia. NPH and regular insulin in combination are available as premixed solutions, which can sometimes simplify administration. The theoretical advantage of glargine and detemir is that they only need to be administered once a day, and they also have steady activity, generally without peaks, although in practice, many patients find that neither lasts a full 24 hours. Glargine and detemir are also signifincantly more expensive, and they cannot be mixed with other forms of insulin.
A short-acting insulin is used to simulate the endogenous insulin surge produced in anticipation of eating. Regular insulin, lispro, aspart and glulisine can be used for this purpose. Regular insulin should be given with about a 30 minute lead-time prior to the meal to be maximally effective and to minimize the possibility of hypoglycemia. Lispro, aspart and glulisine are approved for dosage with the first bite of the meal, and may even be effective if given after completing the meal. The short-acting insulin is also used to correct hyperglycemia.
The usual schedule for checking fingerstick blood glucose and administering insulin is before all meals and sometimes also at bedtime. More recent guidelines also call for a check 2 hours after a meal to ensure the meal has been 'covered' effectively. When insulin glargine or insulin detemir is used, it can be administered at any time during the day, provided that it is given at the same time every day.
Sliding scales
Insulin prescriptions generally specify fixed amounts of long-acting insulin to be given routinely, and fixed amounts of short-acting insulin prior to every meal (the 'sliding scale' approach). However, the amount of short-acting insulin may be varied depending on the patient's preprandial fingerstick glucose, in order to correct pre-existing hyperglycemia. The so-called "sliding-scale" is still widely taught, although it is controversial.
Sample regimen using insulin NPH and regular insulin
| before breakfast | before lunch | before dinner | at bedtime | |
|---|---|---|---|---|
| NPH dose | 12 units | 6 units | ||
| regular insulin dose if fingerstick glucose is (mg/dl): |
||||
| 70-100 | 4 units | 4 units | ||
| 101-150 | 5 units | 5 units | ||
| 151-200 | 6 units | 6 units | ||
| 201-250 | 7 units | 7 units | ||
| 251-300 | 8 units | 1 unit | 8 units | 1 unit |
| >300 | 9 units | 2 units | 9 units | 2 units |
Sample regimen using insulin glargine and insulin lispro
insulin glargine 20 units at bedtime
insulin lispro to be given as follows:
| if fingerstick glucose is (mg/dl): | before breakfast | before lunch | before dinner | at bedtime |
|---|---|---|---|---|
| 70-100 | 5 units | 5 units | 5 units | |
| 101-150 | 6 units | 6 units | 6 units | |
| 151-200 | 7 units | 7 units | 7 units | |
| 201-250 | 8 units | 8 units | 8 units | 1 unit |
| 251-300 | 9 units | 9 units | 9 units | 2 units |
| >300 | 10 units | 10 units | 10 units | 3 units |
Carb counting and DAFNE
A more complicated method that allows greater freedom with meal times and snacks is "carb counting." This approach is taught to diabetic patients in Europe as Dose Adjustment for Normal Eating, or DAFNE. The patient can use his or her total daily dose (TDD) of insulin to estimate how many grams of carbohydrates will be "covered" by 1 unit of insulin, and using this result, the patient can estimate how many units of insulin should be administered depending on the carbohydrate concentration of their meal. For example, if the patient determines that 1 unit of insulin will cover 15 grams of carbohydrates, then they must administer 5 units of insulin before consuming a meal that contains 75 grams of carbohydrates. Some alternative methods also consider the protein content of the meal (since excess dietary protein can be converted to glucose via gluconeogenesis). However, all dosages involve a fair degree of guesswork, and will seldom work consistently from one dosage to the next.
Abuse
There are reports that some patients abuse insulin by injecting large doses that lead to hypoglycemic states. This is extremely dangerous. Severe acute or prolonged hypoglycemia can result in brain damage or death.
On July 23, 2004, news reports claimed that a former spouse of a prominent international track athlete said that the ex-spouse had used insulin as a way of 'energizing' the body. There is no evidence to suggest it should act as a performance enhancer in non-diabetics. Poorly controlled diabetics are more prone than others to exhaustion and tiredness, and properly-administered insulin can relieve such symptoms.
" Game of Shadows," by reporters Mark Fainaru-Wada and Lance Williams, includes allegations that Barry Bonds used insulin in the apparent belief that it would increase the effectiveness of the growth hormone he was (also alleged to be) taking. On top of this, non-prescribed insulin is a banned drug at the Olympics and other global competitions.
The use and abuse of exogenous insulin is reportedly widespread amongst the bodybuilding community. Both insulin, human growth hormone (HGH) and insulin-like growth factor 1 (IGF-1) are self-administered by those looking to increase muscle mass beyond the scope offered by anabolic steroids alone. Their rationale is this: Since insulin and HGH act synergistically to promote growth, and since IGF-1 is the primary mediator of the musculoskeletal effects of growth hormone, the 'stacking' of insulin, HGH and IGF-1 should offer a synergistic growth effect on skeletal muscle. This theory has been borne out in recent years by the creation of top-level bodybuilders whose competition weight is in excess of 50 lb (23 kg) of muscle greater than the professionals of the past, yet with even lower levels of body fat. Indeed, the use of insulin, combined with HGH and/or IGF-1 has resulted in the development of such massively muscled physiques, that there has been a backlash amongst fans of the sport, with a professed disgust at the 'freakish' appearance of top-level professionals.
Bodybuilders will inject up to 10 i.u. of quick-acting synthetic insulin following meals containing starchy carbohydrates and protein, but little fat, in an attempt to 'force feed' nutrients necessary for growth into skeletal muscle, whilst preventing growth of adipocytes. This may be done up to four times each day, following meals, for a total usage of 40iu of synthetic insulin per day. However there have been reports of substantially heavier usage, amongst even 'recreational' bodybuilders.
The abuse of exogenous insulin carries with it an attendant risk of hypoglycemic coma and death. Long-term risks may include development of type 2 diabetes, and potentially a lifetime dependency on exogenous insulin.
History
Discovery and characterization
In 1869 Paul Langerhans, a medical student in Berlin, was studying the structure of the pancreas under a microscope when he identified some previously un-noticed tissue clumps scattered throughout the bulk of the pancreas. The function of the "little heaps of cells," later known as the Islets of Langerhans, was unknown, but Edouard Laguesse later suggested that they might produce secretions that play a regulatory role in digestion. Paul Langerhans' son, Archibald, also helped to understand this regulatory role.
In 1889, the Polish-German physician Oscar Minkowski in collaboration with Joseph von Mering removed the pancreas from a healthy dog to test its assumed role in digestion. Several days after the dog's pancreas was removed, Minkowski's animal keeper noticed a swarm of flies feeding on the dog's urine. On testing the urine they found that there was sugar in the dog's urine, establishing for the first time a relationship between the pancreas and diabetes. In 1901, another major step was taken by Eugene Opie, when he clearly established the link between the Islets of Langerhans and diabetes: Diabetes mellitus … is caused by destruction of the islets of Langerhans and occurs only when these bodies are in part or wholly destroyed. Before his work, the link between the pancreas and diabetes was clear, but not the specific role of the islets.
Over the next two decades, several attempts were made to isolate whatever it was the islets produced as a potential treatment. In 1906 George Ludwig Zuelzer was partially successful treating dogs with pancreatic extract but was unable to continue his work. Between 1911 and 1912, E.L. Scott at the University of Chicago used aqueous pancreatic extracts and noted a slight diminution of glycosuria but was unable to convince his director of his work's value; it was shut down. Israel Kleiner demonstrated similar effects at Rockefeller University in 1919, but his work was interrupted by World War I and he did not return to it. Nicolae Paulescu, a professor of physiology at the University of Medicine and Pharmacy in Bucharest was the first one to isolate insulin, which he called at that time pancrein, and published his work in 1921 that had been carried out in Bucharest. Use of his techniques was patented in Romania, though no clinical use resulted.
In October 1920 Canadian Frederick Banting was reading one of Minkowski's papers and concluded that it is the very digestive secretions that Minkowski had originally studied that were breaking down the islet secretion(s), thereby making it impossible to extract successfully. He jotted a note to himself Ligate pancreatic ducts of the dog. Keep dogs alive till acini degenerate leaving islets. Try to isolate internal secretion of these and relieve glycosurea.
The idea was that the pancreas's internal secretion, which supposedly regulates sugar in the bloodstream, might hold the key to the treatment of diabetes.
He travelled to Toronto to meet with J.J.R. Macleod, who was not entirely impressed with his idea – so many before him had tried and failed. Nevertheless, he supplied Banting with a lab at the University of Toronto, an assistant (medical student Charles Best), and 10 dogs, then left on vacation during the summer of 1921. Their method was tying a ligature (string) around the pancreatic duct, and, when examined several weeks later, the pancreatic digestive cells had died and been absorbed by the immune system, leaving thousands of islets. They then isolated an extract from these islets, producing what they called isletin (what we now know as insulin), and tested this extract on the dogs. Banting and Best were then able to keep a pancreatectomized dog alive all summer because the extract lowered the level of sugar in the blood.
Macleod saw the value of the research on his return but demanded a re-run to prove the method actually worked. Several weeks later it was clear the second run was also a success, and he helped publish their results privately in Toronto, ON that November. However, they needed six weeks to extract the isletin, which forced considerable delays. Banting suggested that they try to use fetal calf pancreas, which had not yet developed digestive glands; he was relieved to find that this method worked well. With the supply problem solved, the next major effort was to purify the extract. In December 1921, Macleod invited the biochemist James Collip, to help with this task, and, within a month, the team felt ready for a clinical test.
On January 11, 1922, Leonard Thompson, a 14-year-old diabetic who lay dying at the Toronto General Hospital, was given the first injection of insulin. However, the extract was so impure that Thompson suffered a severe allergic reaction, and further injections were canceled. Over the next 12 days, Collip worked day and night to improve the ox-pancreas extract, and a second dose was injected on the 23rd. This was completely successful, not only in having no obvious side-effects, but in completely eliminating the glycosuria sign of diabetes.
Children dying from diabetic keto-acidosis were kept in large wards, often with 50 or more patients in a ward, mostly comatose. Grieving family members were often in attendance, awaiting the (until then, inevitable) death. In one of medicine's more dramatic moments Banting, Best and Collip went from bed to bed, injecting an entire ward with the new purified extract. Before they had reached the last dying child, the first few were awakening from their coma, to the joyous exclamations of their families.
However, Banting and Best never worked well with Collip, regarding him as something of an interloper, and Collip left the project soon after.
Over the spring of 1922, Best managed to improve his techniques to the point where large quantities of insulin could be extracted on demand, but the preparation remained impure. The drug firm Eli Lilly and Company had offered assistance not long after the first publications in 1921, and they took Lilly up on the offer in April. In November, Lilly made a major breakthrough, and were able to produce large quantities of highly refined, 'pure' insulin. Insulin was offered for sale shortly thereafter.
Nobel Prizes
The Nobel Prize committee in 1923 credited the practical extraction of insulin to a team at the University of Toronto and awarded the Nobel Prize to two men; Frederick Banting and J.J.R. Macleod. They were awarded the Nobel Prize in Physiology or Medicine in 1923 for the discovery of insulin. Banting, insulted that Best was not mentioned, shared his prize with Best, and Macleod immediately shared his with Collip. The patent for insulin was sold to the University of Toronto for one dollar.
Surprisingly, Banting and Macleod received the 1923 Nobel Prize in Physiology or Medicine for the discovery of insulin, while Paulescu's pioneering work was being completely ignored by the scientific and medical community. International recognition for Paulescu's merits as the true discoverer of insulin came only 50 years later.
The primary structure of insulin was determined by British molecular biologist Frederick Sanger. It was the first protein to have its sequence be determined. He was awarded the 1958 Nobel Prize in Chemistry for this work.
In 1969, after decades of work, Dorothy Crowfoot Hodgkin determined the spatial conformation of the molecule, the so-called tertiary structure, by means of X-ray diffraction studies. She had been awarded a Nobel Prize in Chemistry in 1964 for the development of crystallography.
Rosalyn Sussman Yalow received the 1977 Nobel Prize in Medicine for the development of the radioimmunoassay for insulin.
Timeline of insulin research
- 1922 Banting, Best, Collip use bovine insulin extract in human
- 1923 Eli Lilly produces commercial quantities of much purer bovine insulin than Banting et al had used
- 1923 Farbwerke Hoechst, one of the forerunner's of today's Sanofi Aventis, produces commercial quantities of bovine insulin in Germany
- 1923 Hagedorn founds the Nordisk Insulinlaboratorium in Denmark – forerunner of today's Novo Nordisk
- 1926 Nordisk receives a Danish charter to produce insulin as a non-profit
- 1936 Canadians D.M. Scott, A.M. Fisher formulate a zinc insulin mixture and license it to Novo
- 1936 Hagedorn discovers that adding protamine to insulin prolongs the duration of action of insulin
- 1946 Nordisk formulates Isophane porcine insulin aka Neutral Protamine Hagedorn or NPH insulin
- 1946 Nordisk crystallizes a protamine and insulin mixture
- 1950 Nordisk markets NPH insulin
- 1953 Novo formulates Lente porcine and bovine insulins by adding zinc for longer lasting insulin
- 1955 Frederick Sanger determines the amino acid sequence of insulin
- 1966 Synthesized by total synthesis by C.L. Tsou, Wang Yinglai, and coworkers
- 1969 Dorothy Crowfoot Hodgkin solves the crystal structure of insulin by x-ray crystallography
- 1973 Purified monocomponent (MC) insulin is introduced
- 1973 The U.S. officially "standardized" insulin sold for human use in the U.S. to U-100 (100 units per milliliter). Prior to that, insulin was sold in different strengths, including U-80 (80 units per milliliter) and U-40 formulations (40 units per milliliter), so the effort to "standardize" the potency aimed to reduce dosage errors and ease doctors' job of prescribing insulin for patients. Other countries also followed suit.
- 1978 Genentech produces synthetic 'human' insulin in Escheria coli bacteria using recombinant DNA techniques, licenses to Eli Lilly
- 1981 Novo Nordisk chemically and enzymatically converts porcine to human insulin
- 1982 Genentech synthetic 'human' insulin (above) approved
- 1983 Eli Lilly and Company produces synthetic 'human' insulin with recombinant DNA technology, Humulin
- 1985 Axel Ullrich sequences a human cell membrane insulin receptor.
- 1988 Novo Nordisk produces recombinant human insulin
- 1996 Lilly Humalog "lispro" insulin analogue approved.
- 2000 Sanofi Aventis Lantus "glargine" insulin analogue approved for clinical use in the US and Europe.
- 2004 Sanofi Aventis insulin glulisine insulin analogue approved for clinical use in the US.
- 2006 Novo Nordisk Levemir "detemir" insulin analogue approved for clinical use in the US.