Lyme disease
2008/9 Schools Wikipedia Selection. Related subjects: Health and medicine
| Lyme Disease Classification and external resources |
|
| Nymphal and adult deer ticks can be carriers of Lyme disease. Nymphs are about the size of a poppy seed. | |
| ICD- 9 | 088.81 |
| DiseasesDB | 1531 |
| MedlinePlus | 001319 |
| eMedicine | med/1346 |
Lyme disease, or borreliosis, is an emerging infectious disease caused by at least three species of bacteria belonging to the genus Borrelia. Borrelia burgdorferi is the predominant cause of Lyme disease in the U.S., whereas Borrelia afzelii and Borrelia garinii are implicated in most European cases.
Lyme disease is the most common tick-borne disease in the Northern Hemisphere. The bacteria are transmitted to humans by the bite of infected hard ticks belonging to several species of the genus Ixodes. Early manifestations of infection may include fever, headache, fatigue, and a characteristic skin rash called erythema chronicum migrans. Left untreated, late manifestations involving the joints, heart, and nervous system can occur. In a majority of cases, symptoms can be eliminated with antibiotics, especially if diagnosis and treatment begins early in the course of illness. Late, delayed, or inadequate treatment can lead to "late stage Lyme" or "post-Lyme disease" that can be disabling and difficult to treat.
Symptoms
The Lyme disease bacterium can infect several parts of the body, producing different symptoms at different times. Not all patients with Lyme disease will have all symptoms, and many of the symptoms can occur with other diseases as well.
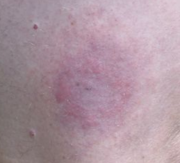
One early sign of infection is a circular rash called erythema chronicum migrans or simply erythema migrans (EM), which occurs at the site of the tick bite. EM is found in 90% of infected people, according to a June, 2008 report. The rash, sometimes referred to as a "bullseye" rash, expands over a period of several days, reaching up to 12 inches (30 cm) across. Most EM lesions remain red throughout or are redder in the centre. Only 9% of EM lesions exhibit the central clearance of the classic bull's eye appearance, however. The rash may be warm but is not usually painful. Some patients develop additional EM lesions in other areas of the body after several days. The disease can progress even in patients who do not develop the rash. Patients may also experience flu-like symptoms of fatigue, chills, fever, headache, muscle and joint aches, and swollen lymph nodes. In some cases, these may be the only symptoms of infection.
If left untreated, the infection may spread to other parts of the body within a few days to weeks, producing an array of discrete symptoms. These include loss of muscle tone on one or both sides of the face (called facial or "Bell's palsy), severe headaches and neck stiffness caused by meningitis, shooting pains that may interfere with sleep, heart palpitations and dizziness caused by changes in heartbeat, and migrating joint pains. Some of these symptoms may resolve, even without treatment.
After several months, untreated or inadequately treated patients may go on to develop severe and chronic symptoms affecting many organs of the body including the brain, nerves, eyes, joints and heart. Shooting pains, numbness or tingling in the hands or feet, problems with concentration and short term memory, severe weakness, vision problems, intolerance to sound and touch, vertigo, back pain, heart block, psychiatric disorders, and swelling of joints are just some of the myriad disabling symptoms that can occur.
The incubation period from infection to the onset of symptoms is usually 1–2 weeks, but can be much shorter (days), or much longer (months to years). Symptoms most often occur from May through September because the nymphal stage of the tick is responsible for most cases. Asymptomatic infection exists but is found in less than 7% of infected individuals in the United States. Asymptomatic infection may be much more common among those infected in Europe.
Other less common findings in acute Lyme disease include cardiac manifestations (up to 10% of patients may have cardiac manifestations including heart block and palpitations), and neurologic symptoms ( neuroborreliosis may occur in up to 18%). In addition, simple altered mental status as the sole presenting symptom has been reported in early neuroborreliosis. Patients have been known to get Baker's cysts.
Post-Lyme disease symptoms
Untreated or inadequately treated cases of Lyme disease may progress to a chronic form most commonly characterized by meningoencephalitis, cardiac inflammation ( myocarditis), frank arthritis, and fatigue. Chronic Lyme disease can have a multitude of symptoms affecting numerous physiological systems: the symptoms appear heterogeneous in the affected population, which may be caused by innate immunity or variations in Borrelia bacteria. Late symptoms of Lyme disease can appear months or years after initial infection and often progress in cumulative fashion over time. Neuropsychiatric symptoms often develop much later in the disease progression, much like tertiary neurosyphilis.
In addition to the acute symptoms, chronic Lyme disease can be manifested by a wide-range of neurological disorders, either central or peripheral, including encephalitis or encephalomyelitis, muscle twitching, polyneuropathy or paresthesia, and vestibular symptoms or other otolaryngologic symptoms, among others. Neuropsychiatric disturbances can occur (possibly from a low-level encephalitis), which may lead to symptoms of memory loss, sleep disturbances, or changes in mood or affect. In rare cases, frank psychosis has been attributed to chronic Lyme disease effects, including mis-diagnoses of schizophrenia and bipolar disorder. Panic attack and anxiety can occur, also delusional behaviour, including somatoform delusions, sometimes accompanied by a depersonalization or derealization syndrome similar to what was seen in the past in the prodromal or early stages of general paresis.
Cause
Lyme disease is caused by Gram-negative spirochetal bacteria from the genus Borrelia. At least 37 Borrelia species have been described, 12 of which are Lyme related. The Borrelia species known to cause Lyme disease are collectively known as Borrelia burgdorferi sensu lato, and have been found to have greater strain diversity than previously estimated.
Until recently it was thought that only three genospecies caused Lyme disease: B. burgdorferi sensu stricto (predominant in North America, but also in Europe), B. afzelii, and B. garinii (both predominant in Eurasia). However, newly discovered genospecies have also been found to cause disease in humans. "There are over 300 strains of Borrelia world wide". It is presently unknown how many of these cause lyme, but some or many of them may.
Transmission
Hard-bodied ticks of the genus Ixodes are the primary vectors of Lyme disease. The majority of infections are caused by ticks in the nymph stage, since adult ticks are more easily detected and removed as a consequence of their relatively large size.
In Europe, the sheep tick, castor bean tick, or European castor bean tick ( Ixodes ricinus) is the transmitter.
In North America, the black-legged tick or deer tick ( Ixodes scapularis) has been identified as the key to the disease's spread on the east coast. Only about 20% of persons infected with Lyme disease by the deer tick are aware of having had any tick bite, making early detection difficult in the absence of a rash. Tick bites often go unnoticed because of the small size of the tick in its nymphal stage, as well as tick secretions that prevent the host from feeling any itch or pain from the bite. The lone star tick ( Amblyomma americanum), which is found throughout the southeastern U.S. as far west as Texas, has been ruled out as a vector of the Lyme disease spirochete Borrelia burgdorferi, though it may be implicated with a clinical syndrome southern tick associated rash illness (STARI), which resembles the skin lesions of Lyme disease.
On the west coast, the primary vector is the western black-legged tick ( Ixodes pacificus). The tendency of this tick species to feed predominantly on host species that are resistant to Borrelia infection appears to diminish transmission of Lyme disease in the West.
While Lyme spirochetes have been found in insects other than ticks, reports of actual infectious transmission appear to be rare. Sexual transmission has been anecdotally reported; Lyme spirochetes have been found in semen and breast milk, however transmission of the spirochete by these routes is not known to occur.
Congenital transmission of Lyme disease can occur from an infected mother to fetus through the placenta during pregnancy, however prompt antibiotic treatment appears to prevent fetal harm.
Tick borne co-infections
Ticks that transmit Lyme Disease also carry and transmit several other parasitic diseases to humans. Thus more physicians are referring to the disease as Lyme and other tick borne illness, and not simply "Lyme".
Babesia infection is becoming more commonly recognized, especially in patients who have Lyme Disease. Ehrlichiosis is another common co-infection found among people with Lyme Disease. (Anaplasma phagocytophila, Human Granulocytic Ehrlichiosis HGE, Human Monocytic Ehrlichiosis HME ) It is also said that Bartonella or Cat Scratch Fever is another common co-infection, although there is debate among experts on this topic on tick to human transmission.
Co-infections complicate Lyme symptoms, especially diagnosis and treatment. It is possible for a tick to carry and transmit one of the co-infections and not Borrelia, making diagnosis difficult and often elusive. The CDC's emerging infections diseases department did a study in rural New Jersey of 100 ticks and found that 55% of the ticks were infected with at least one of the pathogens.
Diagnosis
Lyme disease is diagnosed clinically based on symptoms, objective physical findings (such as erythema migrans, facial palsy, or arthritis), a history of possible exposure to infected ticks, as well as serological tests.
When making a diagnosis of Lyme disease, health care providers should consider other diseases that may cause similar illness. Not all patients with Lyme disease will develop the characteristic bulls-eye rash, and many may not recall a tick bite. Laboratory testing is not recommended for persons who do not have symptoms of Lyme disease.
Because of the difficulty in culturing Borrelia bacteria in the laboratory, diagnosis of Lyme disease is typically based on the clinical exam findings and a history of exposure to endemic Lyme areas. The EM rash is considered sufficient to establish a diagnosis of Lyme disease even when serologies are negative, since it can be observed before the body makes detectable antibodies to Borrelia. Serological testing can be used to support a clinically suspected case.
Diagnosis of late-stage Lyme disease is often difficult because associated symptoms are shared with other diseases. For this reason, Lyme has been called the new "great imitator." Lyme disease may be misdiagnosed as multiple sclerosis, rheumatoid arthritis, fibromyalgia, chronic fatigue syndrome (CFS), lupus, or other autoimmune and neurodegenerative diseases. Conversely, these diseases could be misdiagnosed as Lyme by doctors who do not insist on evidence of Borrelia infection.
Laboratory testing
Several forms of laboratory testing for Lyme disease are available. Most recommended tests are blood tests that measure antibodies made in response to the infection. These tests may be negative in patients with early disease who don't yet have antibodies to Borrelia, but they are quite reliable for diagnosing later stages of disease.
The serological laboratory tests most widely available and employed are the Western blot and the ELISA. A two-tiered protocol is recommended by the CDC: the more sensitive ELISA is performed first, if it is positive or equivocal, the more specific Western blot is run. These tests are sufficiently reliable to support diagnoses, and studies show the Western blot IgM has a specificity of 94–96% for patients with clinical symptoms of early Lyme disease.
Erroneous test results have been widely reported in both early and late stages of the disease. These errors can be caused by several factors, including antibody cross-reactions from other infections including Epstein-Barr virus and cytomegalovirus, as well as herpes simplex virus.
Polymerase chain reaction (PCR) tests for Lyme disease have also been developed to detect the genetic material (DNA) of the Lyme disease spirochete. PCR tests are susceptible to false-positive results from poor laboratory technique. Even when properly performed, PCR often shows false-negative results with blood and CSF specimens. Hence PCR is not widely performed for diagnosis of Lyme disease. However PCR may have a role in diagnosis of Lyme arthritis because it is highly sensitive in detecting ospA DNA in synovial fluid. With the exception of PCR, there is no currently practical means for direct detection of the presence of the organism, as serologic studies only test for antibodies against Borrelia. High titers of either immunoglobulin G (IgG) or immunoglobulin M (IgM) antibodies to Borrelia antigens indicate disease, but lower titers can be misleading. The IgM antibodies may remain after the initial infection, and IgG antibodies may remain for years.
Western blot, ELISA and PCR can be performed by either blood test via venipuncture or cerebrospinal fluid (CSF) via lumbar puncture. Though lumbar puncture is more definitive of diagnosis, antigen capture in the CSF is much more elusive; reportedly CSF yields positive results in only 10-30% of patients cultured. The diagnosis of neurologic infection by Borrelia should not be excluded solely on the basis of normal routine CSF or negative CSF antibody analyses.
New techniques for clinical testing of Borrelia infection have been developed, such as LTT- MELISA, which is capable of identifying the active form of Borrelia infection (Lyme disease). Others, such as focus floating microscopy, are under investigation. New research indicates chemokine CXCL13 may also be a possible marker for neuroborreliosis.
Some laboratories offer Lyme disease testing using assays whose accuracy and clinical usefulness have not been adequately established. These tests include urine antigen tests, immunofluorescent staining for cell wall-deficient forms of Borrelia burgdorferi, and lymphocyte transformation tests. In general, CDC does not recommend these tests.
Imaging
Single photon emission computed tomography (SPECT) imaging has been used to look for cerebral hypoperfusion indicative of Lyme encephalitis in the patient. Although SPECT is not a diagnostic tool itself, it may be a useful method of determining brain function.
In Lyme disease patients, cerebral hypoperfusion of frontal subcortical and cortical structures has been reported. In about 70% of chronic Lyme disease patients with cognitive symptoms, brain SPECT scans typically reveal a pattern of global hypoperfusion in a heterogeneous distribution through the white matter. This pattern is not specific for Lyme disease, since it can also be seen in other central nervous system (CNS) syndromes such as HIV encephalopathy, viral encephalopathy, chronic cocaine use, and vasculitides. However, most of these syndromes can be ruled out easily through standard serologic testing and careful patient history taking.
The presence of global cerebral hypoperfusion deficits on SPECT in the presence of characteristic neuropsychiatric features should dramatically raise suspicion for Lyme encephalopathy among patients who inhabit or have traveled to endemic areas, regardless of patient recall of tick bites. Late disease can occur many years after initial infection. The average time from symptom onset to diagnosis in these patients is about 4 years. Because seronegative disease can occur, and because CSF testing is often normal, Lyme encephalopathy often becomes a diagnosis of exclusion: once all other possibilities are ruled out, Lyme encephalopathy becomes ruled in. Although the aberrant SPECT patterns are caused by cerebral vasculitis, a vasculitide, brain biopsy is not commonly performed for these cases as opposed to other types of cerebral vasculitis.
Abnormal magnetic resonance imaging (MRI) findings are often seen in both early and late Lyme disease. MRI scans of patients with neurologic Lyme disease may demonstrate punctuated white matter lesions on T2-weighted images, similar to those seen in demyelinating or inflammatory disorders such as multiple sclerosis, systemic lupus erythematosus (SLE), or cerebrovascular disease. Cerebral atrophy and brainstem neoplasm has been indicated with Lyme infection as well.
Diffuse white matter pathology can disrupt these ubiquitous gray matter connections and could account for deficits in attention, memory, visuospatial ability, complex cognition, and emotional status. White matter disease may have a greater potential for recovery than gray matter disease, perhaps because neuronal loss is less common. Spontaneous remission can occur in multiple sclerosis, and resolution of MRI white matter hyper-intensities, after antibiotic treatment, has been observed in Lyme disease.
Prevention
Attached ticks should be removed promptly. Protective clothing includes a hat and long-sleeved shirts and long pants that are tucked into socks or boots. Light-colored clothing makes the tick more easily visible before it attaches itself. People should use special care in handling and allowing outdoor pets inside homes because they can bring ticks into your house.
A more effective, community wide method of preventing Lyme disease is to reduce the numbers of primary hosts on which the deer tick depends such as rodents, other small mammals, and deer. Reduction of the deer population may over time help break the reproductive cycle of the deer ticks and their ability to flourish in suburban and rural areas.
Management of host animals
Lyme and all other deer-tick borne diseases can be prevented on a regional level by reducing the deer population that the ticks depend on for reproductive success. This has been effectively demonstrated in the communities of Monhegan, Maine and in Mumford Cove, Connecticut. The black-legged or deer tick (Ixodes scapularis) depends on the white-tailed deer for successful reproduction.
By reducing the deer population back to healthy levels of 8 to 10 per square mile (from the current levels of 60 or more deer per square mile in the areas of the country with the highest Lyme disease rates), the tick numbers can be brought down to very low levels, too few to spread Lyme and other tick-borne diseases.
Vaccination
A vaccine, called Lymerix, against a North American strain of the spirochetal bacteria was approved by the United States FDA on December 21, 1998. It was produced by GlaxoSmithKline (GSK) and was based on the outer surface protein A (OspA) of B. burgdorferi. OspA causes the human immune system to create antibodies that attack that protein.
A group of patients who took Lymerix developed arthritis, muscle pain and other troubling symptoms after vaccination. A class-action lawsuit against GSK was filed on December 14, 1999. On February 26, 2002, GSK decided to withdraw Lymerix from the market citing poor sales, need for frequent boosters, the high price of the vaccine, and the exclusion of children. This was in addition to the numerous financial settlements made because of the vaccine.
While Lymerix was initially being marketed, it was learned that patients with the genetic allele HLA-DR4 were susceptible to T-cell cross-reactivity between epitopes of OspA and lymphocyte function-associated antigen in these patients causing an autoimmune reaction.
New vaccines are being researched using outer surface protein C (OspC) and glycolipoprotein as methods of immunization.
Removal of ticks
Many urban legends exist about the proper and effective method to remove a tick, however it is generally agreed that the most effective method is to pull it straight out with tweezers, making sure not to squeeze the tick or break its head off. Another method is to wrap dental floss around the tick and then pull up to remove it. Gently pinch the tick and drag. Complete removal of the tick head is important; if the head is not completely removed, local infection of bite location may result. However, an alternative method to remove a tick is covering it and surrounding area with an oil thus causing the tick to suffocate. This method is not recommended over standard removal with tweezers, though, as it can irritate the tick and cause it to burrow deeper or regurgitate its stomach contents, increasing the likelihood of disease transmission. Data have demonstrated that prompt removal of an infected tick, within approximately 36 hours, reduces the risk of transmission to nearly zero percent (this has, however, been disproven by a concrete case in Austria where infection resulted after only a few hours) ; however the small size of the tick, especially in the nymph stage, may make detection difficult.
Treatment
Antibiotics are the primary treatment for Lyme disease, but the most appropriate antibiotic treatment depends upon the patient and the stage of the disease. The antibiotics of choice are doxycycline (in adults), amoxicillin (in children), and ceftriaxone. Alternative choices are cefuroxime and cefotaxime.
Results of a recent double blind, randomized, placebo-controlled multicenter clinical study, done in Finland, indicated that oral adjunct antibiotics were not justified in the treatment of patients with disseminated Lyme borreliosis who initially received intravenous antibiotics for 3 weeks. The researchers noted the clinical outcome of said patients should not be evaluated at the completion of intravenous antibiotic treatment but rather 6-12 months afterwards. In patients with chronic post-treatment symptoms, persistent positive levels of antibodies did not seem to provide any useful information for further care of the patient..
In later stages, the bacteria disseminate throughout the body and may cross the blood-brain barrier, making the infection more difficult to treat. Late diagnosed Lyme is treated with oral or IV antibiotics, frequently ceftriaxone, 2 grams per day, for a minimum of four weeks. Minocycline is also indicated for neuroborreliosis for its ability to cross the blood-brain barrier.
Macrolide antibiotics have little efficacy when used alone. One ILADS physician states that combining a macrolide antibiotic such as clarithromycin (biaxin) with hydroxychloroquine (plaquenil) is effective in treatment of "chronic Lyme disease." He suggests that hydroxychloroquine raises the pH of intracellular acidic vacuoles in which B. burgdorferi may reside; raising the pH is thought to activate the macrolide antibiotic, allowing it to inhibit protein synthesis by the spirochete.
Therapies for "post-Lyme syndrome"/"chronic Lyme disease"
Disagreement has arisen in the medical community over the existence and definition of a condition known to proponents of its diagnosis as "chronic Lyme." This condition includes several different patient groups, according to investigators of the Ad Hoc International Lyme Disease Group publishing in the New England Journal of Medicine:
- Patients with symptoms of unknown etiology and no evidence of past or present infection with the Lyme agent
- Patients with a well-defined illness that has nothing to do with Borrelia
- Patients whose symptoms have unknown etiology and have antibodies to Borrelia but no objective signs of Lyme disease
- Patients who have symptoms and have suffered from confirmed Lyme disease
Of these four groups, the majority of patients fall into the first two categories and have been misdiagnosed with "chronic Lyme." Studies that have attempted to enroll patients with persistent Lyme have found that "only 1% to 10% of the screened individuals were eligible," with evidence of past or present Lyme disease. The position of many in the medical community is that only the last group, those who have both symptoms and some evidence of Lyme disease, should be considered Lyme patients, calling this "post-Lyme disease syndrome" if symptoms last for more than six months and "post-Lyme symptoms" if duration is less than six months.
A vocal minority of medical practitioners, many involved with the "International Lyme and Associated Diseases Society" (ILADS), advocates for the diagnosis of "chronic Lyme" in all four patient groups, above. ILADS advocates extended courses of antibiotics, lasting for months, years, or indefinitely, for what it calls chronic Lyme patients. ILADS also advocates for insurance companies to pay their members to administer long-term antibiotic treatments, despite a complete lack of evidence of efficacy.
Scientific consensus, represented by, among others, the American Academy of Neurology, the IDSA, and the Ad Hoc International Lyme Disease Group, states that prolonged treatment with antibiotics has no apparent benefit in treating symptoms that persist following previous standard antibiotic therapy, even in patients with evidence of Lyme disease.
Although the majority of "chronic Lyme" patients do not have any evidence of Lyme disease, there is no reliable evidence that extended antibiotic use is helpful even to those patients who have persistent symptoms after objectively-confirmed Lyme disease. Four randomized, double-blind, placebo-controlled clinical trials have shown that "prolonged antibiotic therapy offers no sustained benefit and has potential serious adverse effects."
- Two studies, published together in 2001, involved one month of intravenous ceftriaxone, followed by two months of oral doxycycline or an identical sequence of IV and oral placebo, given to presumed post-Lyme disease syndrome patients with one or more of the following symptoms: musculoskeletal pain, cognitive impairment, radicular pain, paresthesias or dysesthesias. Two separate analyses were performed, one for Lyme antibody seronegative patients (n=51) and one for Lyme antibody seropositive patients (n=78). No significant benefit was seen in either physical or mental health-related quality of life in either the antibody positive or the antibody negative groups, as assessed with a standardized clinical questionnaire. Two adverse events related to the antibiotic treatment were serious enough to require hospitalization.
- A study published in 2003 tested intravenous ceftriaxone or placebo, given to previously antibiotic-treated Lyme disease patients with "persistent severe fatigue". No improvement was seen in cognitive tests. Modest improvement of fatigue symptoms was observed with the antibiotic treatment. However, antibiotic recipients correctly guessed their assignment more frequently than placebo recipients, suggesting a possible placebo effect. A 7% incidence of severe adverse events (requiring hospitalization) was seen between the two groups, with three episodes of IV sepsis (in the IV placebo group) and one episode of anaphylaxis (in the ceftriaxone group).
- A trial published in 2007 involved ten weeks of intravenous ceftriaxone or placebo given to Borrelia antibody-positive post-Lyme disease syndrome patients with ongoing memory impairment. Neuroimaging techniques recorded patients' responses to treatment at twelve weeks, and assayed the durability of this response at 24 weeks. Although a slight improvement was measured in the ceftriaxone group at twelve weeks, the placebo group also showed improvement, and the difference was not statistically significant unless the treatment group was combined with an aggregate of placebo and untreated, healthy controls. The insignificant differences between treatment and placebo did not persist to 24 weeks. Nine patients had to leave the trial due to adverse effects, including seven treatment-related problems.
Since chronic antibiotic treatments do not work, even for patients with evidence of Lyme disease, most doctors recommend symptomatic treatment for long-term conditions.
Antibiotic-resistant therapies
Antibiotic treatment is the central pillar in the management of Lyme disease. In the late stages of borreliosis, symptoms may persist despite extensive and repeated antibiotic treatment. Lyme arthritis which is antibiotic resistant may be treated with hydroxychloroquine or methotrexate. Experimental data are consensual on the deleterious consequences of systemic corticosteroid therapy. Corticosteroids are not indicated in Lyme disease.
Antibiotic refractory patients with neuropathic pain responded well to gabapentin monotherapy with residual pain after intravenous ceftriaxone treatment in a pilot study. The immunomodulating, neuroprotective and anti-inflammatory potential of minocycline may be helpful in late/chronic Lyme disease with neurological or other inflammatory manifestations. Minocycline is used in other neurodegenerative and inflammatory disorders such as multiple sclerosis, Parkinsons, Huntington's disease, rheumatoid arthritis (RA) and ALS.
Alternative therapies
A number of other alternative therapies have been suggested, though clinical trials have not been conducted. For example, the use of hyperbaric oxygen therapy (which is used conventionally to treat a number of other conditions), as an adjunct to antibiotics for Lyme has been discussed. Though there are no published data from clinical trials to support its use, preliminary results using a mouse model suggest its effectiveness against B. burgdorferi both in vitro and in vivo. Anecdotal clinical research has shown potential for the antifungal azole medications such as diflucan in the treatment of Lyme, but has yet to be repeated in a controlled study or postulated a developed hypothetical model for its use.
Alternative medicine approaches include bee venom because it contains the peptide melittin, which has been shown to exert inhibitory effects on Lyme bacteria in vitro; no clinical trials of this treatment have been carried out, however. The Ad Hoc International Lyme Disease Group warns that some patients have been given "unconventional and highly dangerous" treatments such as bismuth injections or deliberate malaria infection.
Prognosis
For early cases, prompt treatment is usually curative. However, the severity and treatment of Lyme disease may be complicated due to late diagnosis, failure of antibiotic treatment, simultaneous infection with other tick-borne diseases (co-infections), including; ehrlichiosis, babesiosis, and bartonella, and immune suppression in the patient.
A meta-analysis published in 2005 found that some patients with Lyme disease have fatigue, joint and/or muscle pain, and neurocognitive symptoms persisting for years despite antibiotic treatment. Patients with late stage Lyme disease have been shown to experience a level of physical disability equivalent to that seen in congestive heart failure.
In rare cases, Lyme disease can be fatal.The first CDC recognized death from Lyme disease was Amanda Schmidt, age 11.
Ecology
Urbanization and other anthropogenic factors can be implicated in the spread of the Lyme disease into the human population. In many areas, expansion of suburban neighborhoods has led to the gradual deforestation of surrounding wooded areas and increasing "border" contact between humans and tick-dense areas. Human expansion has also resulted in a gradual reduction of the predators that normally hunt deer as well as mice, chipmunks and other small rodents -- the primary reservoirs for Lyme disease. As a consequence of increased human contact with host and vector, the likelihood of transmission to Lyme residents has greatly increased. Researchers are also investigating possible links between global warming and the spread of vector-borne diseases including Lyme disease.
The deer tick (Ixodes scapularis, the primary vector in the northeastern U.S.) has a two-year life cycle, first progressing from larva to nymph, and then from nymph to adult. The tick feeds only once at each stage. In the fall, large acorn forests attract deer as well as mice, chipmunks and other small rodents infected with B. burgdorferi. During the following spring, the ticks lay their eggs. The rodent population then "booms." Tick eggs hatch into larvae, which feed on the rodents; thus the larvae acquire infection from the rodents. (Note: At this stage, it is proposed that tick infestation may be controlled using acaricides ( miticide)).
Adult ticks may also transmit disease to humans. After feeding, female adult ticks lay their eggs on the ground, and the cycle is complete. On the west coast, Lyme disease is spread by the western black-legged tick (Ixodes pacificus), which has a different life cycle.
The risk of acquiring Lyme disease does not depend on the existence of a local deer population, as is commonly assumed. New research suggests that eliminating deer from smaller areas (less than 2.5 ha or 6 acres) may in fact lead to an increase in tick density and the rise of "tick-borne disease hotspots".
Epidemiology
Lyme disease is the most common tick-borne disease in North America and Europe and one of the fastest-growing infectious diseases in the United States. Of cases reported to the United States CDC, the ratio of Lyme disease infection is 7.9 cases for every 100,000 persons. In the ten states where Lyme disease is most common, the average was 31.6 cases for every 100,000 persons for the year 2005.
Although Lyme disease has now been reported in 49 of 50 states in the U.S, about 99% of all reported cases are confined to just five geographic areas (New England, Mid-Atlantic, East-North Central, South Atlantic, and West North-Central) . New 2008 CDC Lyme case definition guidelines are used to determine confirmed CDC surveillance cases. Effective January 2008, the CDC gives equal weight to laboratory evidence from 1) a positive culture for B. burgdorferi; 2) two-tier testing (ELISA screening and Western Blot confirming); or 3) single-tier IgG (old infection) Western Blot. Previously, the CDC only included laboratory evidence based on (1) and (2) in their surveillance case definition. The case definition now includes the use of Western Blot without prior ELISA screen.
The number of reported cases of the disease have been increasing, as are endemic regions in North America. For example, it had previously been thought that B. burgdorferi sensu lato was hindered in its ability to be maintained in an enzootic cycle in California because it was assumed the large lizard population would dilute the prevalence of B. burgdorferi in local tick populations, but this has since been brought into question as some evidence has suggested that lizards can become infected. Except for one study in Europe , much of the data implicating lizards is based on DNA detection of the spirochete and has not demonstrated that lizards are able to infect naive ticks feeding upon them . As some experiments suggest lizards are refractory to infection with Borrelia, it appears likely their involvement in the enzootic cycle is more complex and species-specific .
While B. burgdorferi is most associated with deer tick and the white tailed mouse, Borrelia afzelii is most frequently detected in rodent-feeding vector ticks, Borrelia garinii and Borrelia valaisiana appear to be associated with birds. Both rodents and birds are competent reservoir hosts for B. burgdorferi sensu stricto. The resistance of a genospecies of Lyme disease spirochetes to the bacteriolytic activities of the alternative complement pathway of various host species may determine its reservoir host association.
In Europe, cases of B. burgdorferi sensu lato infected ticks are found predominantly in Norway, Netherlands, Germany, France, Italy, Slovenia and Poland, but have been isolated in almost every country on the continent .
B. burgdorferi sensu lato infested ticks are being found more frequently in Japan, as well as in Northwest China and far eastern Russia. Borrelia has been isolated in Mongolia as well.
In South America tick-borne disease recognition and occurrence is rising. Ticks carrying B. burgdorferi sensu lato, as well as canine and human tick-borne disease, have been reported widely in Brazil, but the subspecies of Borrelia has not yet been defined. The first reported case of Lyme disease in Brazil was made in 1993 in Sao Paulo. B. burgdorferi sensu stricto antigens in patients have been identified in Colombia and Bolivia.
In Northern Africa B. burgdorferi sensu lato has been identified in Morocco, Algeria, Egypt and Tunisia.
Lyme disease in sub-Saharan is presently unknown, but evidence indicates that Lyme disease may occur in humans in this region. The abundance of hosts and tick vectors would favour the establishment of Lyme infection in Africa. In East Africa, two cases of Lyme disease have been reported in Kenya.
In Australia there is no definitive evidence for the existence of B. burgdorferi or for any other tick-borne spirochete that may be responsible for a local syndrome being reported as Lyme disease. Cases of neuroborreliosis have been documented in Australia but are often ascribed to travel to other continents. The existence of Lyme disease in Australia is controversial.
To date, data shows that Northern hemisphere temperate regions are most endemic for Lyme disease.
Controversy and politics
When the Infectious Diseases Society of America (IDSA) issued new guidelines for Lyme diagnosis in 2006, recommending actual evidence of Lyme before diagnosis and treatment, the ILADS and allied chronic Lyme advocacy organizations quickly condemned the guidelines. Connecticut Attorney General Richard Blumenthal, who had received awards from chronic Lyme advocacy groups, began a probe into the IDSA panel responsible for the guidelines, putatively on anti-trust grounds. On May 1st, 2008, Blumenthal announced news of a settlement in the case..
Although Blumenthal alleged conflicts of interest on the part of IDSA, he declined to name the allegedly conflicted panelists or detail what he considered their conflict to be. The IDSA panel responded by stating they had been wrongly "accused of profiting financially by recommending to not treat with unnecessary and prolonged courses of antibiotics," while IDSA president Dr. Donald Poretz disputed the presence of conflicts and was confident the treatment guidelines were correct. Under the settlement, IDSA will appoint a new, independent panel of doctors and scientists to conduct a complete review of the IDSA guidelines.
Advancing immunology research
Long term persistence of T cell lymphocyte responses to B. burgdorferi as an "immunological scar syndrome" was hypothesized in 1990. The role of Th1 and interferon-gamma (IFN-gamma) in borrelia was first described in 1995. The cytokine pattern of Lyme disease, and the role of Th1 with down regulation of interleukin-10 (IL-10) was first proposed in 1997.
Inflammation
Recent studies in both acute and antibiotic refractory, or chronic, Lyme disease have shown a distinct pro- inflammatory immune process. This pro-inflammatory process is a cell-mediated immunity and results in Th1 upregulation. These studies have shown a significant decrease in cytokine output of (IL-10), an upregulation of Interleukin-6 (IL-6), Interleukin-12 (IL-12) and IFN-gamma and disregulation in TNF-alpha predominantly.
These studies suggest that the host immune response to infection results in increased levels of IFN-gamma in the serum and lesions of Lyme disease patients that correlate with greater severity of disease. IFN-gamma alters gene expression by endothelia exposed to B. burgdorferi in a manner that promotes recruitment of T cells and suppresses that of neutrophils.
Studies also suggest suppressors of cytokine signaling (SOCS) proteins are induced by cytokines, and T cell receptor can down-regulate cytokine and T cell signaling in macrophages. It is hypothesized that SOCS are induced by IL-10 and B. burgdorferi and its lipoproteins in macrophages, and that SOCS may mediate the inhibition of IL-10 by concomitantly elicited cytokines. IL-10 is generally regarded as an anti-inflammatory cytokine, since it acts on a variety of cell types to suppress production of proinflammatory mediators.
Researchers are also beginning to identify microglia as a previously unappreciated source of inflammatory mediator production following infection with B. burgdorferi. Such production may play an important role during the development of cognitive disorders in Lyme neuroborreliosis. This effect is associated with induction of nuclear factor-kappa B (NF-KB) by Borrelia.
Disregulated production of pro-inflammatory cytokines such as IL-6 and TNF-alpha can lead to neuronal damage in Borrelia infected patients. IL-6 and TNF-Alpha cytokines produce fatigue and malaise, two of the more prominent symptoms experienced by patients with chronic Lyme disease.IL-6 is also significantly indicated in cognitive impairment.
Neuroendocrine
A developing hypothesis is that the chronic secretion of stress hormones as a result of Borrelia infection may reduce the effect of neurotransmitters, or other receptors in the brain by cell-mediated pro-inflammatory pathways, thereby leading to the dysregulation of neurohormones, specifically glucocorticoids and catecholamines, the major stress hormones. This process is mediated via the Hypothalamic-pituitary-adrenal axis. Additionally Tryptophan, a precursor to serotonin appears to be reduced within the CNS in a number of infectious diseases that affect the brain, including Lyme. Researchers are investigating if this neurohormone secretion is the cause of neuro-psychiatric disorders developing in some patients with borreliosis.
Antidepressants acting on serotonin, norepinephrine and dopamine receptors have been shown to be immunomodulatory and anti-inflammatory against pro-inflammatory cytokine processes, specifically on the regulation of IFN-gamma and IL-10, as well as TNF-alpha and IL-6 through a psycho-neuroimmunological process. Antidepressants have also been shown to suppress Th1 upregulation.These studies warrant investigation for antidepressants for use in a psycho-neuroimmunological approach for optimal pharmacotherapy of antibiotic refractory Lyme patients.
New developments
New research has also found that chronic Lyme patients have higher amounts of Borrelia-specific forkhead box P3 (FoxP3) than healthy controls, indicating that regulatory T cells might also play a role, by immunosuppression, in the development of chronic Lyme disease. FoxP3 are a specific marker of regulatory T cells. The signaling pathway P38 mitogen-activated protein kinases (p38 MAP kinase) has also been identified as promoting expression of pro-inflammatory cytokines from Borrelia.
The culmination of these new and ongoing immunological studies suggest this cell-mediated immune disruption in the Lyme patient amplifies the inflammatory process, often rendering it chronic and self-perpetuating, regardless of whether the Borrelia bacterium is still present in the host, or in the absence of the inciting pathogen in an autoimmune pattern. This interpretation must however be considered against the evidence (above) for persistence of the 'spore' form of Borrelia in human and animal hosts, and the tendency for relapses to occur after antibiotics are continued. It is possible that whereas some chronic Lyme patients retain actual populations of live spirochaetes, others have symptoms brought on only by an inflammatory or auto-immune reaction.
Researchers hope that this new developing understanding of the biomolecular basis and pathology of cell-mediated signaling events caused by B. burgdorferi infection will lead to a greater understanding of immune response and inflammation caused by Lyme disease and, hopefully, new treatment strategies for chronic antibiotic-resistant disease.
History
The early European studies of what is now known as Lyme disease described its various skin manifestations. The first such study dates to 1883 in Wrocław, Poland (then known as Breslau, Germany) where physician Alfred Buchwald described a man who had suffered for sixteen years with a degenerative skin disorder now known as acrodermatitis chronica atrophicans. At a 1909 research conference, Swedish dermatologist Arvid Afzelius presented a study about an expanding, ring-like lesion he had observed in an older woman following the bite of a sheep tick. He named the lesion erythema migrans. The skin condition now known as borrelial lymphocytoma was first described in 1911.
Neurological problems following tick bites were recognized starting in the 1920s. French physicians Garin and Bujadoux described a farmer with a painful sensory radiculitis accompanied by mild meningitis following a tick bite. A large ring-shaped rash was also noted, although the doctors did not relate it to the meningoradiculitis. In 1930, the Swedish dermatologist Sven Hellerstrom was the first to propose that EM and neurological symptoms following a tick bite were related. In the 1940s, German neurologist Alfred Bannwarth described several cases of chronic lymphocytic meningitis and polyradiculoneuritis, some of which were accompanied by erythematous skin lesions.
Carl Lennhoff, who worked at the Karolinska Institute in Sweden, believed that many skin conditions were caused by spirochetes. In 1948, he used a special stain to microscopically observe what he believed were spirochetes in various types of skin lesions, including EM. Although his conclusions were later shown to be erroneous, interest in the study of spirochetes was sparked. In 1949, Nils Thyresson, who also worked at the Karolinska Institute, was the first to treat ACA with penicillin. In the 1950s, the relationship among tick bite, lymphocytoma, EM and Bannwarth's syndrome were recognized throughout Europe leading to the widespread use of penicillin for treatment in Europe.
In 1970 a dermatologist in Wisconsin named Rudolph Scrimenti recognized an EM lesion in a patient after recalling a paper by Hellerstrom that had been reprinted in an American science journal in 1950. This was the first documented case of EM in the United States. Based on the European literature, he treated the patient with penicillin.
The full syndrome now known as Lyme disease was not recognized until a cluster of cases originally thought to be juvenile rheumatoid arthritis was identified in three towns in southeastern Connecticut in 1975, including the towns Lyme and Old Lyme, which gave the disease its popular name. This was investigated by Dr David Snydman and Dr Allen Steere of the Epidemic Intelligence Service, and by others from Yale University. The recognition that the patients in the United States had EM led to the recognition that "Lyme arthritis" was one manifestation of the same tick-borne condition known in Europe.
Before 1976, elements of B. burgdorferi sensu lato infection were called or known as tickborne meningopolyneuritis, Garin-Bujadoux syndrome, Bannworth syndrome, Afzelius syndrome, Montauk Knee or sheep tick fever. Since 1976 the disease is most often referred to as Lyme disease, Lyme borreliosis or simply borreliosis.
In 1980, Allen Steere and colleagues began to test antibiotic regimens in adult patients with Lyme disease In 1982 a novel spirochete was cultured from the mid-gut of Ixodes ticks in Shelter Island, New York, and subsequently from patients with Lyme disease. The infecting agent was then identified by Jorge Benach at the State University of New York at Stony Brook, and soon after isolated by Willy Burgdorfer, a researcher at the National Institutes of Health, who specialized in the study of arthropod-borne bacteria such as Borrelia and Rickettsia. The spirochete was named Borrelia burgdorferi in his honour. Burgdorfer was the partner in the successful effort to culture the spirochete, along with Alan Barbour.
After identification B. burgdorferi as the causative agent of Lyme disease, antibiotics were selected for testing, guided by in vitro antibiotic sensitivities, including tetracycline antibiotics, amoxicillin, cefuroxime axetil, intravenous and intramuscular penicillin and intravenous ceftriaxone. The mechanism of tick transmission was also the subject of much discussion. B. burgdorferi spirochetes were identified in tick saliva in 1987, confirming the hypothesis that transmission occurred via tick salivary glands.