Phase (matter)
2008/9 Schools Wikipedia Selection. Related subjects: Physics
In the physical sciences, a phase is a set of states of a macroscopic physical system that have relatively uniform chemical composition and physical properties (i.e. density, crystal structure, index of refraction, and so forth).
Phases vs. states of matter
Phases are sometimes confused with states of matter, but there are significant differences. States of matter refers to the differences between gases, liquids, solids, plasma, etc. If there are two regions in a chemical system that are in different states of matter, then they must be different phases. However, the reverse is not true -- a system can have multiple phases which are in equilibrium with each other and also in the same state of matter. This difference is especially important when considering the Gibbs' phase rule, which governs the number of allowed phases.
Mixtures can have multiple phases, which often happen when two immiscible substances dissolve into one another in small amounts. For example, a mixture might be composed of an oil phase (95% oil, 5% water) and a water phase (95% water, 5% oil).
Polymorphism is the ability of a solid to exist in more than one crystal form. For example, water ice is ordinarily found in the hexagonal form Ice Ih, but can also exist as the cubic ice Ic, the rhombohedral ice II, and many other forms.
Amorphous phases are also possible with the same molecule, such as amorphous ice. In this case, the phenomenon is known as polyamorphism.
For pure chemical elements, polymorphism is known as allotropy. For example, diamond, graphite, and fullerenes are different allotropes of carbon.
General definition of phases
In general, two different states of a system are in different phases if there is an abrupt change in their physical properties while transforming from one state to the other. Conversely, two states are in the same phase if they can be transformed into one another without any abrupt changes. There are, however, exceptions to this statement -- for example the liquid-gas critical point discussed below in the Phase Diagrams section.
An important point is that different types of phases are associated with different physical qualities. When discussing the solid, liquid, and gaseous phases, we talked about rigidity and compressibility, and the effects of varying the pressure and volume, because those are the relevant properties that distinguish a solid, a liquid, and a gas. On the other hand, when discussing paramagnetism and ferromagnetism, we look at the magnetization, because that is what distinguishes the ferromagnetic phase from the paramagnetic phase. Several more examples of phases will be given in the following section.
In more technical language, a phase is a region in the parameter space of thermodynamic variables in which the free energy is analytic; between such regions there are abrupt changes in the properties of the system, which correspond to discontinuities in the derivatives of the free energy function. As long as the free energy is analytic, all thermodynamic properties (such as entropy, heat capacity, magnetization, and compressibility) will be well-behaved, because they can be expressed in terms of the free energy and its derivatives. For example, the entropy is the negative of the first derivative of the free energy with temperature (at constant pressure).
When a system goes from one phase to another, there will generally be a stage where the free energy is non-analytic. This is a phase transition. Due to this non-analyticity, the free energies on either side of the transition are two different functions, so one or more thermodynamic properties will behave very differently after the transition. The property most commonly examined in this context is the heat capacity. During a transition, the heat capacity may become infinite, jump abruptly to a different value, or exhibit a "kink" or discontinuity in its derivative. See also differential scanning calorimetry.
Phase diagrams
The different phases of a system may be represented using a phase diagram. The axes of the diagrams are the relevant thermodynamic variables. For simple mechanical systems, we generally use the pressure and temperature.
The markings on the phase diagram show the points where the free energy is non-analytic. The open spaces, where the free energy is analytic, correspond to the phases. The phases are separated by lines of non-analyticity, where phase transitions occur, which are called phase boundaries.
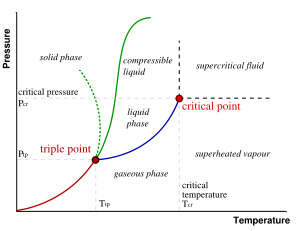
In the diagram, the phase boundary between liquid and gas does not continue indefinitely. Instead, it terminates at a point on the phase diagram called the critical point. At temperatures and pressure above the critical point, the physical property differences that differentiate the liquid phase from the gas phase become less defined. This reflects the fact that, at extremely high temperatures and pressures, the liquid and gaseous phases become indistinguishable. In water, the critical point occurs at around 647 K (374 °C or 705 °F) and 22.064 MPa.
The existence of the liquid-gas critical point reveals a slight ambiguity in our above definitions. When going from the liquid to the gaseous phase, one usually crosses the phase boundary, but it is possible to choose a path that never crosses the boundary by going to the right of the critical point. Thus, phases can sometimes blend continuously into each other. This new phase which has some properties that are similar to a liquid and some properties that are similar to a gas is called a supercritical fluid. We should note, however, that this does not always happen. For example, it is impossible for the solid-liquid phase boundary to end in a critical point in the same way as the liquid-gas boundary, because the solid and liquid phases have different symmetry.
An interesting thing to note is that the solid-liquid phase boundary in the phase diagram of most substances, such as the one shown above, has a positive slope. This is due to the solid phase having a higher density than the liquid, so that increasing the pressure increases the melting temperature. However, in the phase diagram for water the solid-liquid phase boundary has a negative slope. This reflects the fact that ice has a lower density than water, which is an unusual property for a material.
Phase separation
Phase separation is transformation of a homogenous system into two (or more) phases and commonly encountered in many branches of science and technology. One example is the crystallization of a solid from a solution. A universal mathematical model of phase separation is provided by the Cahn–Hilliard equation.
Phase equilibrium
The distribution of kinetic energy among molecules is not uniform, and it changes randomly. This means that at, say, the surface of a liquid, there may be an individual molecule with enough kinetic energy to jump into the gas phase. Likewise, individual gas molecules may have low enough kinetic energy to join other molecules in the liquid phase. This phenomenon means that at any given temperature and pressure, multiple phases may co-exist.
For example, under standard conditions for temperature and pressure, a bowl of liquid water in dry air will evaporate until the partial pressure of gaseous water equals the vapor pressure of water. At this point, the rate of molecules leaving and entering the liquid phase becomes the same (due to the increased number of gaseous water molecules available to re-condense). The fact that liquid molecules with above-average kinetic energy have been removed from the bowl results in evaporative cooling. Similar processes may occur on other types of phase boundaries.
Gibbs' phase rule relates the number of possible phases, variables such as temperature and pressure, and whether or not an equilibrium will be reached.
Phase transition
A phase transition or, phase change, describes when a substance changes its state of matter - ex. ice melting to water is a phase change because a solid changed to a liquid. For a phase change to occur, energy must be added or removed from the substance. The heat energy, or enthalpy, associated with a solid to liquid transition is the enthalpy of fusion, that for liquid to gas is the enthalpy of vaporization, and for solid to gas is the heat of sublimation. Normally adding or removing energy will change the temperature of the substance as the kinetic energy of the particles will increase or decrease. During a phase change however, the potential energy of the substance changes as the particles are moved further apart or closer together. There is no change in kinetic energy of the particles and therefore no resulting change in temperature.