From Wikipedia, the free encyclopedia
 Size of this preview: 446 × 480 pixels
Size of this preview: 446 × 480 pixels Full resolution (1,762 × 1,895 pixels, file size: 862 KB, MIME type: image/png)
 |
This is a file from the Wikimedia Commons. The description on its description page there is shown below.Commons is a freely licensed media file repository. You can help.
|
 |
This is a featured picture, which means that members of the community have identified it as one of the finest images on the English Wikipedia, adding significantly to its accompanying article. If you have a different image of similar quality, be sure to upload it using the proper free license tag, add it to a relevant article, and nominate it. |
 |
This image was selected as picture of the day on the English Wikipedia for June 28, 2006. |
allotropes of carbon
 |
This is a featured picture on English Wikipedia and is considered one of the finest images.
- If you think this file should be featured on Wikimedia Commons as well, feel free to nominate it.
- If you have an image of similar quality that can be published under a suitable copyright license, be sure to upload it, tag it, and nominate it.
|
 |
Created by Michael Ströck (mstroeck) on Februar 7, 2006 using iMol for Mac OS X and Photoshop CS2. Released under the GFDL.
| Description |
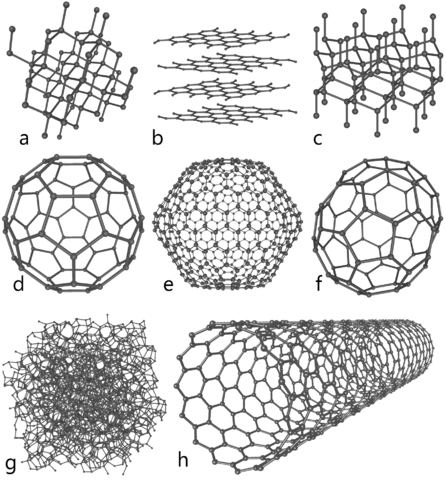
English: This illustration depicts eight of the allotropes (different molecular configurations) that pure carbon can take:
- a) Diamond
- b) Graphite
- c) Lonsdaleite
- d) C60 ( Buckminsterfullerene)
- e) C540 (see Fullerene)
- f) C70 (see Fullerene)
- g) Amorphous carbon
- h) single-walled carbon nanotube
Polski: Odmiany węgla:
- a) diament
- b) grafit
- c) Lonsdaleit
- d) fuleren C60
- e) fuleren C540
- f) fuleren C70
- g) węgiel amorficzny
- h) nanorurka
|
| Source |
Created by Michael Ströck (mstroeck) |
| Date |
Februar 7, 2006 |
| Author |
Created by Michael Ströck (mstroeck) |
Permission
( Reusing this image) |
GFDL
|
Licensing
I, the copyright holder of this work, hereby publish it under the following licenses:
 |
Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation license, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. A copy of the license is included in the section entitled " GNU Free Documentation license".
Aragonés | العربية | Asturianu | Беларуская (тарашкевіца) | Български | বাংলা | ইমার ঠার/বিষ্ণুপ্রিয়া মণিপুরী | Brezhoneg | Bosanski | Català | Cebuano | Česky | Dansk | Deutsch | Ελληνικά | English | Esperanto | Español | Eesti | Euskara | فارسی | Suomi | Français | Gaeilge | Galego | עברית | Hrvatski | Magyar | Bahasa Indonesia | Ido | Íslenska | Italiano | 日本語 | ქართული | ភាសាខ្មែរ | 한국어 | Kurdî / كوردی | Latina | Lëtzebuergesch | Lietuvių | Bahasa Melayu | Nnapulitano | Nederlands | Norsk (nynorsk) | Norsk (bokmål) | Occitan | Polski | Português | Română | Русский | Slovenčina | Slovenščina | Shqip | Српски / Srpski | Svenska | తెలుగు | ไทย | Tagalog | Türkçe | Українська | اردو | Tiếng Việt | Volapük | Yorùbá | 中文(简体) | 中文(繁體) | +/- |
You may select the license of your choice.
|
File history
Click on a date/time to view the file as it appeared at that time.
|
|
Date/Time |
Dimensions |
User |
Comment |
| current |
20:51, 11 May 2008 |
1,762×1,895 (862 KB) |
Mahahahaneapneap |
|
|
|
05:48, 7 October 2007 |
1,762×1,895 (871 KB) |
Whkoh |
|
|
|
01:22, 11 April 2007 |
1,762×1,895 (849 KB) |
CountingPine |
|
|
|
13:37, 18 February 2006 |
1,762×1,895 (1.48 MB) |
Mstroeck |
|
File links
The following pages on Schools Wikipedia link to this image (list may be incomplete):

