International Union of Pure and Applied Chemistry nomenclature
2008/9 Schools Wikipedia Selection. Related subjects: Chemistry
IUPAC nomenclature is a system of naming chemical compounds and of describing the science of chemistry in general. It is developed and kept up to date under the auspices of the International Union of Pure and Applied Chemistry (IUPAC).
The rules for naming organic and inorganic compounds are contained in two publications, known as the Blue Book and the Red Book respectively. A third publication, known as the Green Book, describes the recommendations for the use of symbols for physical quantities (in association with the IUPAP), while a fourth, the Gold Book, contains the definitions of a large number of technical terms used in chemistry. Similar compendia exist for biochemistry (in association with the IUBMB), analytical chemistry and macromolecular chemistry . These books are supplemented by shorter recommendations for specific circumstances which are published from time to time in the journal Pure and Applied Chemistry.
This article treats the system of nomenclature in general, notably its aims and historical development. Separate articles treat the naming of organic compounds and inorganic compounds in more detail.
Aims of chemical nomenclature
The primary function of chemical nomenclature is to ensure that the person who hears or reads a chemical name is under no ambiguity as to which chemical compound it refers: each name should refer to a single substance. It is considered less important to ensure that each substance should have a single name, although the number of acceptable names is limited.
It is also preferable that the name convey some information about the structure or chemistry of a compound. CAS numbers form an extreme example of names which do not perform this function: each refers to a single compound but none contain information about the structure. One might be tempted to add [7647-14-5] to one's meal, but not [133-43-9]—the former is sodium chloride, the latter sodium cyanide.
The form of nomenclature which should be used depends on the public to which it is addressed: as such there is no single correct form, but rather different forms which are more or less appropriate in different circumstances.
A common name will often suffice to identify a chemical compound in a particular set of circumstances. There is little risk that the "salt" on a dinner table will be sodium cyanide (technically a salt itself). To be more generally applicable, the name should indicate at least the chemical formula: hence table salt is referred to chemically as sodium chloride, which indicates by the rules of inorganic nomenclature that the formula is NaCl. To be more specific still, the three-dimensional arrangement of the atoms may need to be specified: there are occasions where it might be necessary to distinguish between sodium chloride (halite structure) (the common form) and cesium chloride (CsCl structure) (of theoretical interest only).
In a few specific circumstances (such as the construction of large indices), it becomes necessary to ensure that each compound has a unique name: this requires the addition of extra rules to the standard IUPAC system (the CAS system is the most commonly used in this context), at the expense of having names which are longer and less familiar to most readers. Another system gaining popularity is the International Chemical Identifier — while InChI symbols are not human readable, they contain complete information about substance structure. That makes them more general than CAS numbers.
The IUPAC system is often criticized for the above failures when they become relevant (for example in differing reactivity of sulfur allotropes which IUPAC doesn't distinguish). While IUPAC has a human-readable advantage over CAS numbering, it would be difficult to claim that the IUPAC names for some larger, relevant molecules (such as rapamycin) are human-readable, and so most researchers simply use the informal names.
History
The nomenclature of alchemy is rich in description, but does not effectively meet the aims outlined above. Opinions differ whether this was deliberate on the part of the early practitioners of alchemy or whether it was a consequence of the particular (and often esoteric) theoretical framework in which they worked.
While both explanations are probably valid to some extent, it is remarkable that the first "modern" system of chemical nomenclature appeared at the same time as the distinction (by Lavoisier) between elements and compounds, in the late eighteenth century.
The French chemist Louis-Bernard Guyton de Morveau published his recommendations in 1782, hoping that his "constant method of denomination" would "help the intelligence and relieve the memory". The system was refined in collaboration with Berthollet, de Fourcroy and Lavoisier, and promoted by the latter in a textbook which would survive long after his death at the guillotine in 1794. The project was also espoused by Jöns Jakob Berzelius, who adapted the ideas for the German-speaking world.
The recommendations of Guyton covered only what would be today known as inorganic compounds. With the massive expansion of organic chemistry in the mid-nineteenth century and the greater understanding of the structure of organic compounds, the need for a less ad hoc system of nomenclature was felt just as the theoretical tools became available to make this possible. An international conference was convened in Geneva in 1892 by the national chemical societies, from which the first widely accepted proposals for standardization arose.
A commission was set up in 1913 by the Council of the International Association of Chemical Societies, but its work was interrupted by World War I. After the war, the task passed to the newly formed International Union of Pure and Applied Chemistry, which first appointed commissions for organic, inorganic and biochemical nomenclature in 1921 and continues to do so to this day.
Types of nomenclature
Compositional nomenclature
e.g.hydrogen
Stock nomenclature/Classical
Stock nomenclature is used for naming inorganic compounds, and is based on the indication of the oxidation state of the metal cation.
To name a compound, some simple rules are followed:
- The cationic element is named first.
- The oxidation state of the cation is then given, as Roman numerals in parentheses, if it is multivalent.
- The anionic element or group is then named. See list of common anion names
For example, for FeCl2:
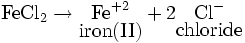
The name of FeCl2 is: iron(II) chloride.
