Sodium chloride
2008/9 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Sodium chloride | |
|---|---|
 |
|
 |
|
| IUPAC name | Sodium chloride |
| Other names | Common salt; halite; table salt |
| Identifiers | |
| CAS number | [7647-14-5] |
| RTECS number | VZ4725000 |
| Properties | |
| Molecular formula | NaCl |
| Molar mass | 58.442 g/mol |
| Appearance | White or colorless crystals or powder |
| Density | 2.16 g/cm³, solid |
| Melting point |
801 °C |
| Boiling point |
1465 °C (1738 K) |
| Solubility in water | 35.9 g/100 mL (25 °C) |
| Structure | |
| Coordination geometry |
Octahedral |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant and might sting |
| NFPA 704 | |
| R-phrases | 36 |
| S-phrases | none |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | NaF, NaBr, NaI |
| Other cations | LiCl, KCl, RbCl, CsCl, MgCl2, CaCl2 |
| Related salts | Sodium acetate |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references |
|
Sodium chloride, also known as common salt, table salt, or halite, is a chemical compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms. As the major ingredient in edible salt, it is commonly used as a condiment and food preservative. In one gram of sodium chloride, there are approximately 0.3933 grams of sodium, and 0.6067 grams of chlorine.
Production and use
Salt is currently mass produced by evaporation of seawater or brine from other sources, such as brine wells and salt lakes, and by mining rock salt, called halite. In 2002, world production was estimated at 210 million metric tonnes, the top five producers being the United States (40.3 million tonnes), China (32.9), Germany (17.7), India (14.5), and Canada (12.3).
As well as the familiar uses of salt in cooking, salt is used in many applications, from manufacturing pulp and paper to setting dyes in textiles and fabric, to producing soaps and detergents. In cold countries, large quantities of rock salt are used to help clear highways of ice during winter, although "Road Salt" loses its melting ability at temperatures below -15°C to -20°C (5°F to -4°F). Sodium chloride is sometimes used as a cheap and safe desiccant due to its hygroscopic properties, making salting an effective method of food preservation historically. Even though more effective desiccants are available, few are safe for humans to ingest.
| Solubility of NaCl in various solvents (g NaCl / 100 g of solvent at 25 °C) |
|
|---|---|
| H2O | 26 |
| Liquid ammonia | 3.02 |
| Methanol | 1.4 |
| Formic acid | 5.2 |
| Sulfolane | 0.005 |
| Acetonitrile | 0.0003 |
| Acetone | 0.000042 |
| Formamide | 9.4 |
| Dimethylformamide | 0.04 |
| Reference: Burgess, J. Metal Ions in Solution (Ellis Horwood, New York, 1978) ISBN 0-85312-027-7 |
|
Synthetic uses
Salt is also the raw material used to produce chlorine which itself is required for the production of many modern materials including PVC and pesticides. Industrially, elemental chlorine is usually produced by the electrolysis of sodium chloride dissolved in water. Along with chlorine, this chloralkali process yields hydrogen gas and sodium hydroxide, according to the chemical equation
- 2NaCl + 2H2O → Cl2 + H2 + 2NaOH
Sodium metal is produced commercially through the electrolysis of liquid sodium chloride. This is done in a Down's cell in which sodium chloride is mixed with calcium chloride to lower the melting point below 700 °C. As calcium is more electropositive than sodium, no calcium will be formed at the cathode. This method is less expensive than the previous method of electrolyzing sodium hydroxide.
Sodium chloride is used in other chemical processes for the large-scale production of compounds containing sodium or chlorine. In the Solvay process, sodium chloride is used for producing sodium carbonate and calcium chloride. In the Mannheim process and in the Hargreaves process, it is used for the production of sodium sulfate and hydrochloric acid.
Biological uses
Many microorganisms cannot live in an overly salty environment: water is drawn out of their cells by osmosis. For this reason salt is used to preserve some foods, such as smoked bacon or fish and can also be used to detach leeches that have attached themselves to feed. It has also been used to disinfect wounds. In medieval times salt would be rubbed into household surfaces as a cleansing agent.
Biological functions
In humans, a high-salt intake was demonstrated to attenuate Nitric Oxide production. Nitric oxide (NO) contributes to vessel homeostasis by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to the endothelium [ ]
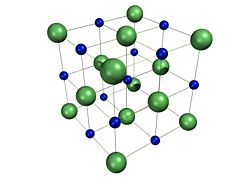
Crystal structure
Sodium chloride forms crystals with cubic symmetry. In these, the larger chloride ions, shown to the right as green spheres, are arranged in a cubic close-packing, while the smaller sodium ions, shown to the right as blue spheres, fill the octahedral gaps between them.
Each ion is surrounded by six ions of the other kind. This same basic structure is found in many other minerals, and is known as the halite structure. This arrangement is known as cubic close packed (ccp). It can be represented as two interpenetrating face-centered cubic (fcc) lattices, or one fcc lattice with a two atom basis. It is most commonly known as the rocksalt crystal structure.
It is held together with an ionic bond and electrostatic forces.
Road salt
While salt was once a scarce commodity in history, industrialized production has now made salt plentiful. About 51% of world output is now used by cold countries to de-ice roads in winter, both in grit bins and spread by winter service vehicles. This works because salt and water form an eutectic mixture. Adding salt to water will lower the freezing temperature of the water, depending on the concentration. The salinity of water is measured as grams salt per kilogram (1000g) water, and the freezing temperatures are as follows.
| S(g/kg) | 0 | 10 | 20 | 24.7 | 30 | 35 |
| T(freezing) (C) | 0 | -0.5 | -1.08 | -1.33 | -1.63 | -1.91 |
Much of the road salt used in Europe comes from mines in Carrickfergus.
Additives
Table salt sold for consumption today is not pure sodium chloride. In 1911 magnesium carbonate was first added to salt to make it flow more freely. In 1924 trace amounts of iodine in form of sodium iodide, potassium iodide or potassium iodate were first added, to reduce the incidence of simple goiter.
Salt for de-icing in the UK typically contains sodium hexacyanoferrate (II) at less than 100ppm as an anti-caking agent. In recent years this additive has also been used in table salt.
Common chemicals
Chemicals used in de-icing salts are mostly found to be sodium chloride (NaCl) or calcium chloride (CaCl2). Both are similar and are effective in de-icing roads. When these chemicals are produced, they are mined/made, crushed to fine granules, then treated with an anti-caking agent. Adding salt lowers the freezing point of the water, which allows the liquid to be stable at lower temperatures and allows the ice to melt. Alternative de-icing chemicals have also been used. Chemicals such as calcium magnesium acetate and potassium formate are being produced. These chemicals have few of the negative chemical effects on the environment commonly associated with NaCl and CaCl2.