Platinum
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements; Mineralogy
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | platinum, Pt, 78 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 10, 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | grayish white |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 195.084 (9) g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d9 6s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 17, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 21.45 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 19.77 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2041.4 K (1768.3 ° C, 3214.9 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4098 K (3825 ° C, 6917 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 22.17 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 469 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 25.86 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic face centered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 1, 2, 3, 4, 5, 6 (mildly basic oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.28 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 870 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1791 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 135 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 177 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 128 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 175 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 105 n Ω·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 71.6 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 8.8 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) 2800 m·s−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 168 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 61 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 230 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4–4.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 549 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 392 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-06-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Platinum (pronounced /ˈplætɪnəm/) is a chemical element with the atomic symbol Pt and an atomic number of 78. It is in group 10 of the Periodic Table of Elements. A heavy, malleable, ductile, precious, gray-white transition metal, platinum is resistant to corrosion and occurs in some nickel and copper ores along with some native deposits. Platinum is used in jewelry, laboratory equipment, electrical contacts, dentistry, and automobile emissions control devices. Platinum bullion has the ISO currency code of XPT.
Notable characteristics
When pure, the metal appears greyish-white and firm. The metal is corrosion-resistant. The catalytic properties of the six platinum family metals are outstanding. For this catalytic property, platinum is used in catalytic converters, incorporated in automobile exhaust systems, as well as tips of spark plugs.
Platinum's wear- and tarnish-resistance characteristics are well suited for making fine jewelry. Platinum is more precious than gold. The price of platinum changes along with its availability, but its price is normally more than 200% of the price of gold. In the 18th century, platinum's rarity made King Louis XV of France declare it the only metal fit for a king. Platinum possesses high resistance to chemical attack, excellent high-temperature characteristics, and stable electrical properties. All these properties have been exploited for industrial applications. Platinum does not oxidize in air at any temperature, but can be corroded by cyanides, halogens, sulfur, and caustic alkalis. This metal is insoluble in hydrochloric and nitric acid, but does dissolve in the mixture known as aqua regia (forming chloroplatinic acid). Common oxidation states of platinum include +2, and +4. The +1 and +3 oxidation states are less common, and are often stabilized by metal bonding in bimetallic (or polymetallic) species.The gold is removed from this solution as a precipitate by treatment with iron(II) chloride (FeCl2). The platinum is precipitated out as impure (NH4)2PtCl6 on treatment with NH4Cl, leaving H2PtCl4 in solution.
Applications
- As a catalyst in the catalytic converter, an optional (though often mandatory by law) component of the gasoline-fueled automobile exhaust system (see "Notable characteristics" in this article).
- As a catalyst in fuel cells. Reducing the amount of platinum required (and thus cost) is a major focus of fuel cell research.
- Certain platinum-containing compounds are capable of crosslinking DNA and kill cells by similar pathways to alkylating chemotherapeutic agents. Cisplatin, carboplatin and oxaliplatin are licensed examples of this class of drugs.
- Platinum resistance thermometers.
- Electrodes for use in electrolysis and electrochemical measurements (e.g., the standard hydrogen electrode).
- In the Clark polarographic electrode for measuring oxygen tension.
- A wide range of jewelry.
- As a catalyst in the curing of silicone elastomers.
- As a catalyst in glow plugs in some model engines.
- In crucibles, alloyed with rhodium (10–40% of Rh), for high temperature melting (around 1500°C) of glass.
- In photography, it is sometimes used for archival printmaking. Platinum prints display a greater range of tones than other Black and White printing methods. Additionally platinum's chemical stability makes for extremely long-lasting prints. The disadvantage of this method, in addition to the high cost, is that platinum is less light sensitive and prints must be contact printed at the same size as the negative. Therefore, enlargements can only be made by making an enlarged negative. Platinum salts alone generally create excessive contrast in prints; combined with salts from its sister metal, palladium, produce warmer and softer tones, without diminishing the tonal range platinum enables.
- In watchmaking, Vacheron Constantin, Patek Philippe, Rolex, Breitling and other companies use platinum for producing their limited edition watch series. Watchmakers highly appreciate the unique properties of platinum as it neither tarnishes nor wears out.
History
Naturally-occurring platinum and platinum-rich alloys have been known for a long time. Though the metal was used by pre-Columbian Native Americans, the first European reference to platinum appears in 1557 in the writings of the Italian humanist Julius Caesar Scaliger ( 1484– 1558) as a description of a mysterious metal found in Central American mines between Darién (Panama) and Mexico ("up until now impossible to melt by any of the Spanish arts"). The word platinum comes from the Spanish word platina, meaning "little silver."
Platinum was discussed by astronomer Antonio de Ulloa and Don Jorge Juan y Santacilia ( 1713–1773), both appointed by King Philip V to join a geographical expedition in Peru that lasted from 1735 to 1745. Amongst other things, Ulloa observed the platina del pinto, the unworkable metal found with gold in New Granada (Colombia). British privateers intercepted Ulloa's ship on the return voyage. Though he was well-treated in England, and even made a member of the Royal Society he was prevented from publishing a reference to the unknown metal until 1748. Before that could happen Charles Wood independently isolated the element in 1741. Major finds were discovered in Russia in 1819, which produced around 90% of the global Platinum production at the turn of the 20th century.
Due to its rarity, greater difficulty to work with and the need to alloy it with (at the time) an even more expensive metal iridium, platinum was only used in a limited way in jewelry at the end of the 19th century. This changed at beginning of the 20th century when most diamond ring mountings and most exclusive jewelry were almost completely made of platinum. From 1875 to 1960 the SI unit of length (the standard metre) was defined as the distance between two lines on a standard bar of an alloy of ninety percent platinum and ten percent iridium, measured at 0 degrees Celsius.
Occurrence
Platinum is an extremely rare metal, occurring as only 0.003 ppb in the Earth's crust, and is 30 times rarer than gold. If all the world's platinum reserves were poured into one Olympic-size swimming pool, it would be just deep enough to cover one's ankles. Gold would fill more than three such pools.
In 2005, South Africa was the top producer of platinum with almost 80% share followed by Russia and Canada, reports the British Geological Survey.
Platinum is often found chemically uncombined as native platinum and alloyed with iridium as platiniridium. The platinum arsenide, sperrylite (PtAs2), is a major source of platinum associated with nickel ores in the Sudbury Basin deposit in Ontario, Canada. The rare sulfide mineral cooperite, (Pt,Pd,Ni)S, contains platinum along with palladium and nickel. Cooperite occurs in the Merensky Reef within the Bushveld complex, Gauteng, South Africa.
Platinum, often accompanied by small amounts of other platinum family metals, occurs in alluvial placer deposits in the Witwatersrand of South Africa, the Ural Mountains, and in the Absaroka Range in the American state of Montana, the only place it is found in the western hemisphere.
Platinum is produced commercially as a by-product of nickel ore processing in the Sudbury deposit. The huge quantities of nickel ore processed makes up for the fact that platinum is present as only 0.5 ppm in the ore.
Platinum exists in relatively higher abundances on the Moon and in meteorites. Corrospondingly, platinum is found in slightly higher abundances at sites of bollide impact on the Earth that are associated with resulting post-impact volcanism, and can be mined economically; the Sudbury Basin is one such example.
Precautions
According to the Centers for Disease Control and Prevention, short-term exposure to platinum salts "may cause irritation of the eyes, nose, and throat" and long-term exposure "may cause both respiratory and skin allergies." The current OSHA standard is 0.002 milligram per cubic meter of air averaged over an 8-hour work shift.
Certain platinum complexes are used in chemotherapy and show good anti-tumor activity for some tumours. Cisplatin is particularly effective against testicular cancer; cure rate was improved from 10% to 85%. However, the side effects are severe. Cisplatin causes cumulative, irreversible kidney damage and deafness.
As platinum is a catalyst in the manufacture of the silicone rubber and gel components of several types of medical implants (breast implants, joint replacement prosthetics, artificial lumbar discs, vascular access ports), the possibility that platinum free radicals could enter the body and cause adverse effects has merited study. The FDA and other countries have reviewed the issue and found no evidence to suggest toxicity in vivo.
Rarity and colour
Platinum's rarity as a metal has caused advertisers to associate it with exclusivity and wealth. "Platinum" credit cards have greater privileges than do "gold" ones. "Platinum awards" are the second highest possible, ranking above gold, silver and bronze, but below "Diamond". For example, in the United States a musical album that has sold more than 1,000,000 copies, will be credited as "platinum", whereas an album that sold more than 10,000,000 copies will be certified as “diamond”. And some products, such as blenders and vehicles, with a silvery-white colour are identified as "platinum". Platinum is considered a precious metal, although its use is not as common as the use of gold or silver. The frame of the Crown of Queen Elizabeth the Queen Mother, manufactured for her Coronation as Consort of King George VI, is made of platinum. It was the first British crown to be made of this particular metal.
Production
In order to obtain pure platinum, the ore is crushed, made into a slurry, and then mixed with a detergent containing "collector" molecules. Air is then blown through the mixture, enabling the grains of metal minerals to be separated from the rest of the mixture. This process is called " flotation." The next step is smelting.
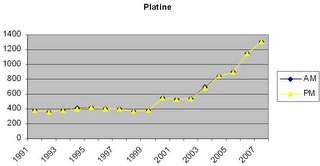
In 2006, world supply of platinum was of about 217,700 kg or 7 million troy ounces.
Average Price from 1991 to 2007 in $ per troy ounce (~$40/g).