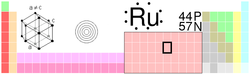
Ruthenium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | Ruthenium, Ru, 44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 8, 5, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white metallic |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 101.07 (2) g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d7 5s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 15, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 12.45 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 10.65 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2607 K (2334 ° C, 4233 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4423 K (4150 ° C, 7502 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 38.59 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 591.6 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 24.06 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 8, 6, 4, 3, 2, 1, (mildly acidic oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.2 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 710.2 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1620 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2747 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 130 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 178 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 126 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 71 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 117 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 6.4 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 5970 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 447 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 173 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 220 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 2160 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-18-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ruthenium (pronounced /ruːˈθiːniəm/) is a chemical element that has the symbol Ru and atomic number 44. A rare transition metal of the platinum group of the periodic table, ruthenium is found associated with platinum ores and used as a catalyst in some platinum alloys.
Notable characteristics
A polyvalent hard white metal, ruthenium is a member of the platinum group, has four crystal modifications and does not tarnish at normal temperatures, but does oxidize readily on exposure to air to form ruthenium tetroxide, RuO4, a strong oxidising agent with properties analogous to those of osmium tetroxide. Ruthenium dissolves in fused alkalis, is not attacked by acids but is attacked by halogens at high temperatures. Small amounts of ruthenium can increase the hardness of platinum and palladium. The corrosion resistance of titanium is increased markedly by the addition of a small amount of ruthenium.
This metal can be plated either through electrodeposition or by thermal decomposition methods. One ruthenium-molybdenum alloy has been found to be superconductive at 10.6 K. The oxidation states of ruthenium range from +1 to +8, and -2 is known, though oxidation states of +2, +3, and +4 are most common.
Applications
Due to its ability to harden platinum and palladium, ruthenium is used in platinum and palladium alloys to make wear-resistant electrical contacts. It is sometimes alloyed with gold in jewelry. 0.1% ruthenium is added to titanium to improve its corrosion resistance a hundredfold.
Ruthenium is also used in some advanced high-temperature single-crystal superalloys, with applications including the turbine blades in jet engines.
Fountain pen nibs are frequently tipped with alloys containing ruthenium. From 1944 onward, the famous Parker 51 fountain pen was fitted with the "RU" nib, a 14K gold nib tipped with 96.2% ruthenium and 3.8% iridium.
Ruthenium is also a versatile catalyst. Hydrogen sulfide can be split by light by using an aqueous suspension of CdS particles loaded with ruthenium dioxide. This may be useful in the removal of H2S from oil refineries and from other industrial processes.
Ruthenium is a component of mixed-metal oxide (MMO) anodes used for cathodic protection of underground and submerged structures, and for electrolytic cells for chemical processes such as generating chlorine from saltwater.
Organometallic ruthenium carbene and allenylidene complexes have recently been found as highly efficient catalysts for olefin metathesis with important applications in organic and pharmaceutical chemistry.
Some ruthenium complexes absorb light throughout the visible spectrum and are being actively researched in various, potential, solar energy technologies. Ruthenium-based dyes have been used as the electron providers in dye-sensitized solar cells, a promising new low-cost solar cell system.
The fluorescence of some ruthenium complexes is quenched by oxygen, which has led to their use as optode sensors for oxygen.
Ruthenium red, [(NH3)5Ru-O-Ru(NH3)4-O-Ru(NH3)5]6+, is a biological stain used to stain polyanionic molecules such as pectin and nucleic acids for light microscopy and electron microscopy.
Ruthenium-centered complexes are being researched for possible anticancer properties. Ruthenium, unlike traditional platinum complexes, show greater resistance to hydrolysis and more selective action on tumors. NAMI-A and KP1019 are two drugs undergoing clinical evaluation against metastatic tumors and colon cancers.
Applications of Ruthenium Thin Films in Microelectronics
Relatively recently, ruthenium has been suggested as a material that could beneficially replace other metals and silicides in microelectronics components. Ruthenium tetroxide (RuO4) is highly volatile, as is ruthenium trioxide (RuO3). By oxidizing ruthenium (for example with an oxygen plasma) into the volatile oxides, ruthenium can be easily patterned. The properties of the common ruthenium oxides make ruthenium a metal compatible with the semiconductor processing techniques needed to manufacture microelectronics.
In order to continue miniaturization of microelectronics, new materials are needed as dimensions change. There are three main applications for thin ruthenium films in microelectronics. The first is using thin films of ruthenium as electrodes on both sides of tantalum pentoxide (Ta2O5) or barium strontium titanate ((Ba, Sr)TiO3, also known as BST) in the next generation of three-dimensional dynamic random access memories ( DRAMs). Ruthenium thin film electrodes could also be deposited on top of lead zirconate titanate (Pb(ZrxTi1-x)O3, also known as PZT) in another kind of RAM, ferroelectric random access memory ( FRAM). Platinum has been used as the electrodes in RAMs in laboratory settings, but it is difficult to pattern. Ruthenium is chemically similar to platinum, preserving the function of the RAMs, but in contrast to Pt patterns easily. The second is using thin ruthenium films is as metal gates in p-doped metal-oxide-semiconductor field effect transistors (p-MOSFETs). When replacing silicide gates with metal gates in MOSFETs, a key property of the metal is its work function. The work function needs to match the surrounding materials. For p-MOSFETs, the ruthenium work function is the best materials property match with surrounding materials such as HfO2, HfSiOx, HfNOx, and HfSiNOx, to achieve the desired electrical properties. The third large-scale application for ruthenium films is as a combination adhesion promoter and electroplating seed layer between TaN and Cu in the copper dual damascene process. Copper can be directly electroplated onto ruthenium, in contrast to tantalum nitride. Copper also adheres poorly to TaN, but well to Ru. By depositing a layer of ruthenium on the TaN barrier layer, copper adhesion would be improved and deposition of a copper seed layer would not be necessary.
There are also other uses suggested. In 1990, IBM scientists discovered that a thin layer of ruthenium atoms created a strong anti-parallel coupling between adjacent ferromagnetic layers, stronger than any other nonmagnetic spacer-layer element. Such a ruthenium layer was used in the first giant magnetoresistive read element for hard disk drives. In 2001, IBM announced a three-atom-thick layer of the element ruthenium, informally referred to as pixie dust, which would allow a quadrupling of the data density of current hard disk drive media.
History
Ruthenium was discovered and isolated by Russian scientist Karl Klaus in 1844 in Kazan University, Kazan. Klaus showed that ruthenium oxide contained a new metal and obtained 6 grams of ruthenium from the part of crude platinum that is insoluble in aqua regia.
Jöns Berzelius and Gottfried Osann nearly discovered ruthenium in 1827. The men examined residues that were left after dissolving crude platinum from the Ural Mountains in aqua regia. Berzelius did not find any unusual metals, but Osann thought he found three new metals and named one of them ruthenium.
The name derives from Ruthenia, the Latin word for Rus', a historical area which includes present-day western Russia, Ukraine, Belarus, and parts of Slovakia and Poland. Karl Klaus named the element in honour of his birthland, as he was born in Tartu, Estonia, which was at the time a part of the Russian Empire.
It is also possible that Polish chemist Jędrzej Śniadecki isolated element 44 (which he called vestium) from platinum ores in 1807. However his work was never confirmed, and he later withdrew his claim of discovery.
Occurrence
Normal mining
This element is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, Canada, and in pyroxenite deposits in South Africa.
Ruthenium is exceedingly rare and is the 74th most abundant metal on earth [Nature's Building Block, John Emsley, Oxford University Press,2001]. Roughly 12MT of Ru is mined each year with world reserves estimated to be 5000mt [Nature's Building Block, John Emsley, Oxford University Press,2001].
This metal is commercially isolated through a complex chemical process in which hydrogen is used to reduce ammonium ruthenium chloride yielding a powder. The powder is then consolidated by powder metallurgy techniques or by argon- arc welding.
From used nuclear fuels
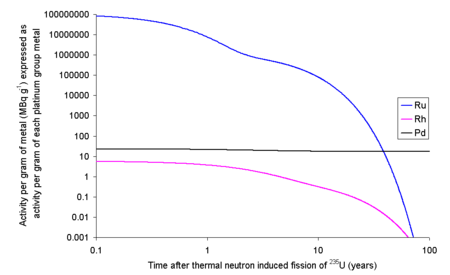
It is also possible to extract ruthenium from used nuclear fuel. Each kilo of fission products of 235U will contain 63.44 grams of ruthenium isotopes with halflives longer than a day. Since a typical used nuclear fuel contains about 3% fission products, one ton of used fuel will contain about 1.9 kg of ruthenium. The 103Ru and 106Ru will render the fission ruthenium very radioactive. If the fission occurs in an instant then the ruthenium thus formed will have an activity due to 103Ru of 109 TBq g-1 and 106Ru of 1.52 TBq g-1. Ru 103 has a half life of about 39 days meaning that within 390 days it will have effectively decayed to ground state, well before any reprocessing is likely to occur. Ru 106 has a half life of about 373 days meaning that if the fuel is let to cool for 5 years before reprocessing only about 3% of the original quantity will remain, the rest will have decayed to ground state.
Compounds
Ruthenium compounds are often similar in properties to those of osmium and exhibit at least eight oxidation states, but the +2, +3, and +4 states are the most common. Examples are ruthenium(IV) oxide (Ru(IV)O2, oxidation state +4), dipotassium ruthenate (K2Ru(VI)O4, +6), potassium perruthenate (KRu(VII)O4, +7) and ruthenium tetroxide (Ru(VIII)O4, +8). Compounds of ruthenium with chlorine are ruthenium(II) chloride (RuCl2) and ruthenium(III) chloride (RuCl3).
Isotopes
Naturally occurring ruthenium is composed of seven isotopes. The most stable radioisotopes are 106Ru with a half-life of 373.59 days, 103Ru with a half-life of 39.26 days and 97Ru with a half-life of 2.9 days.
Fifteen other radioisotopes have been characterized with atomic weights ranging from 89.93 u (90Ru) to 114.928 u (115Ru). Most of these have half-lives that are less than five minutes except 95Ru (half-life: 1.643 hours) and 105Ru (half-life: 4.44 hours).
The primary decay mode before the most abundant isotope, 102Ru, is electron capture and the primary mode after is beta emission. The primary decay product before 102Ru is technetium and the primary mode after is rhodium.
Organometallic chemistry
Ruthenium is a versatile metal that can easily It is quite easy to form compounds with carbon ruthenium bonds, as these compounds tend to be darker and react more quickly than the osmium compounds. Recently, Professor Anthony Hill and his co-workers have been making compounds of ruthenium in which a boron atom binds to the metal atom.
The organometallic ruthenium compound that is easiest to make is RuHCl(CO)(PPh3)3. This compound has two forms (yellow and pink) that are identical once they are dissolved but different in the solid state.
An organometallic compound similar to ruthenocene, bis(2,4-dimethylpentadienyl)ruthenium, is readily synthesized in near quantitative yields and has applications in vapor-phase deposition of metallic ruthenium, as well as in catalysis, including Fischer-Tropsch synthesis of transportation fuels.
Important catalysts based on ruthenium are Grubbs' catalyst and Roper's complex.
Chemical Vapor Deposition of Ruthenium
A unique challenge arises in trying to grow impurity-free films of a catalyst. Ruthenium metal activates C-H and C-C bonds, which aids C-H and C-C bond scission. This creates a potential catalytic decomposition path for all metal-organic CVD precursors that is likely to lead to significant carbon incorporation. Platinum, a chemically similar catalyst, catalyzes dehydrogenation of five- and six-member cyclic hydrocarbons into benzene. The d-bands of ruthenium lie higher than those in platinum, generally predicting stronger ruthenium-adsorbate bonds than on platinum.21 Therefore, it is likely that ruthenium also catalyzes dehydrogenation of five- and six-member hydrocarbon rings to benzene. Benzene dehydrogenates further on ruthenium surfaces into hydrocarbon fragments similar to those formed by acetylene and ethene on ruthenium surfaces. In addition to benzene, acetylene and ethene, pyridine also decomposes on ruthenium surfaces, leaving bound fragments on the surface.
Ruthenium is unusually well studied in the surface science and catalysis literature due to its industrial importance as a catalyst. There are many studies of individual molecular behavior on ruthenium in surface science. However, understanding the behavior of each ligand on its own is not equivalent to understanding their behaviour when co-adsorbed with each other and with the precursor. While there is no significant pressure difference between surface science studies and CVD, there is often a temperature gap between temperatures reported in surface science studies and CVD growth temperatures. Despite these complications, ruthenium is a promising candidate for understanding chemical vapor deposition and precursor design of catalytic films.
Ligands that are stable compounds in their own right, short ligand-ruthenium contact times and moderate substrate temperatures help minimize unwanted ligand decomposition on the surface. The C-H and C-C bond activation is temperature-dependent. Product desorption is also temperature-dependent, if the products are not bound to the ruthenium surface. This suggests that there is some optimum temperature, at which most independently stable ligands have just enough thermal energy to desorb from the ruthenium film surface before C-H activation can occur. For example, benzene starts decomposing on ruthenium at 87°C. However, the dehydrogenation reaction does not go to fragments until 277°C, and compete fragmentation is not seen at low surface coverages. This suggests that provided adsorbed benzene molecules are not close to one another on the surface and temperatures are below 277°C, the vast majority of benzene molecules may not contribute to carbon incorporation in films. Therefore, a key consideration in growing CVD films of catalytic metals such as ruthenium is combining molecule design and the kinetic aspects of growth in a favorable way.
Before metal-organic precursors were explored, triruthenium dodecacarbonyl (Ru3(CO)12) was tested as a CVD precursor. While this precursor gives good-quality films, the vapor pressure is poor, complicating its practical use in a CVD process. Ruthenocene and bis(ethylcyclopentadienyl)ruthenium(II) and beta-diketonate ruthenium(II) compounds have been fairly extensively explored. Although these precursors also can give pure films of low resistivity when reacted with oxygen, the growth rates are very low or not reported. One high-growth precursor, cyclopentadienyl-propylcyclopentadienylruthenium(II) (RuCp(i-PrCp)), has been identified. (RuCp(i-PrCp) has achieved growth rates of 7.5nm/min to 20 nm/min as well as low resistivities. However, it does not nucleate on oxides, ruling out its use in all applications but copper interconnect playing layers.
A new zero-valent, single-source precursor design paradigm was launched by Schneider et al with (1,5-cyclooctadiene)(toluene)Ru(0) ((1,5-COD)(toluene)Ru) and (1,3-cyclohexadiene)(benzene)Ru(0) ((1,3-CHD)(benzene)Ru), also independently tested by Choi et al. Using (1,5-COD)(toluene)Ru, Schneider found that C-H bonds were readily activated in 1,5-COD. Although carbon incorporation levels were low (1-3%), the growth rates were only around 0.28nm/min at best. Using (1,3-CHD)(benzene)Ru, the 1,3-CHD was dehydrogenated to benzene as expected, but the large variety of possible surface reactions involving the two ligands resulted in a narrow process window in which carbon concentrations were low.
Precautions
The compound ruthenium tetroxide, RuO4, similar to osmium tetroxide, is volatile, highly toxic and may cause explosions if allowed to come into contact with combustible materials. Ruthenium plays no biological role but does strongly stain human skin, may be carcinogenic and bio-accumulates in bone.