Vitamin C
2008/9 Schools Wikipedia Selection. Related subjects: Agriculture; Chemical compounds
 |
|
 |
|
|
Vitamin C
|
|
| Systematic (IUPAC) name | |
| 2-oxo-L-threo-hexono-1,4- lactone-2,3-enediol or (R)-3,4-dihydroxy-5-((S)- 1,2-dihydroxyethyl)furan-2(5H)-one |
|
| Identifiers | |
| CAS number | |
| ATC code | A |
| PubChem | |
| Chemical data | |
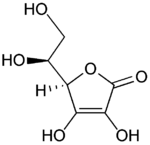
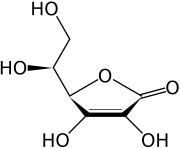
| Formula | C6H8O6 |
| Mol. mass | 176.14 grams per mol |
| Synonyms | L-ascorbate |
| Physical data | |
| Melt. point | 190–192 °C (374–378 °F) decomposes |
| Pharmacokinetic data | |
| Bioavailability | rapid & complete |
| Protein binding | negligible |
| Metabolism | ? |
| Half life | 30 minutes |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. |
A |
| Legal status |
general public availability |
| Routes | oral |
Vitamin C or L-ascorbate is an essential nutrient for a large number of higher primate species, a small number of other mammalian species (notably guinea pigs and bats), a few species of birds, and some fish.
The presence of ascorbate is required for a range of essential metabolic reactions in all animals and plants. It is made internally by almost all organisms, humans being the most well-known exception. It is widely known as the vitamin whose deficiency causes scurvy in humans. It is also widely used as a food additive.
The pharmacophore of vitamin C is the ascorbate ion. In living organisms, ascorbate is an antioxidant, since it protects the body against oxidative stress, and is a cofactor in several vital enzymatic reactions.
The uses and the daily requirement amounts of vitamin C are matters of on-going debate. People consuming diets rich in ascorbate from natural foods, such as fruits and vegetables, are healthier and have lower mortality from a number of chronic illnesses. However, a recent meta-analysis of 68 reliable antioxidant supplementation experiments involving a total of 232,606 individuals concluded that consuming additional ascorbate from supplements may not be as beneficial as thought.
Biological significance
Vitamin C is purely the L-enantiomer of ascorbate; the opposite D-enantiomer has no physiological significance. Both forms are mirror images of the same molecular structure. When L-ascorbate, which is a strong reducing agent, carries out its reducing function, it is converted to its oxidized form, L-dehydroascorbate. L-dehydroascorbate can then be reduced back to the active L-ascorbate form in the body by enzymes and glutathione.
L-ascorbate is a weak sugar acid structurally related to glucose which naturally occurs either attached to a hydrogen ion, forming ascorbic acid, or to a metal ion, forming a mineral ascorbate.
Function
In humans, vitamin C is a highly effective antioxidant, acting to lessen oxidative stress, a substrate for ascorbate peroxidase, as well as an enzyme cofactor for the biosynthesis of many important biochemicals. Vitamin C acts as an electron donor for eight different enzymes:
- Three participate in collagen hydroxylation. These reactions add hydroxyl groups to the amino acids proline or lysine in the collagen molecule (via prolyl hydroxylase and lysyl hydroxylase), thereby allowing the collagen molecule to assume its triple helix structure and making vitamin C essential to the development and maintenance of scar tissue, blood vessels, and cartilage.
- Two are necessary for synthesis of carnitine. Carnitine is essential for the transport of fatty acids into mitochondria for ATP generation.
- The remaining three have the following functions:
- dopamine beta hydroxylase participates in the biosynthesis of norepinephrine from dopamine.
- another enzyme adds amide groups to peptide hormones, greatly increasing their stability.
- one modulates tyrosine metabolism.
Biological tissues that accumulate over 100 times the level in blood plasma of vitamin C are the adrenal glands, pituitary, thymus, corpus luteum, and retina. Those with 10 to 50 times the concentration present in blood plasma include the brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands.
Biosynthesis
The vast majority of animals and plants are able to synthesize their own vitamin C, through a sequence of four enzyme-driven steps, which convert glucose to vitamin C. The glucose needed to produce ascorbate in the liver (in mammals and perching birds) is extracted from glycogen; ascorbate synthesis is a glycogenolysis-dependent process. In reptiles and birds the biosynthesis is carried out in the kidneys.
Among the animals that have lost the ability to synthesise vitamin C are simians (specifically the suborder haplorrhini), guinea pigs, a number of species of passerine birds (but not all of them), and in apparently many major families of bats and perhaps all of them. Humans have no enzymatic capability to manufacture vitamin C. The cause of this phenomenon is that the last enzyme in the synthesis process, L-gulonolactone oxidase, cannot be made by the listed animals because the gene for this enzyme, Pseudogene ΨGULO, is defective. The mutation has not been lethal because vitamin C is abundant in their food sources. It has been found that species with this mutation (including humans) have adapted a vitamin C recycling mechanism to compensate.
Most simians consume the vitamin in amounts 10 to 20 times higher than that recommended by governments for humans. This discrepancy constitutes the basis of the controversy on current recommended dietary allowances.
It has been noted that the loss of the ability to synthesize ascorbate strikingly parallels the evolutionary loss of the ability to break down uric acid. Uric acid and ascorbate are both strong reducing agents. This has led to the suggestion that in higher primates, uric acid has taken over some of the functions of ascorbate. Ascorbic acid can be oxidised (broken down) in the human body by the enzyme ascorbic acid oxidase.
An adult goat, a typical example of a vitamin C-producing animal, will manufacture more than 13,000 mg of vitamin C per day in normal health and the biosynthesis will increase "many fold under stress". Trauma or injury has also been demonstrated to use up large quantities of vitamin C in humans. Some microorganisms such as the yeast Saccharomyces cerevisiae have been shown to be able to synthesize vitamin C from simple sugars.
Deficiency
Scurvy is an avitaminosis resulting from lack of vitamin C, since without this vitamin, the synthesised collagen is too unstable to perform its function. Scurvy leads to the formation of liver spots on the skin, spongy gums, and bleeding from all mucous membranes. The spots are most abundant on the thighs and legs, and a person with the ailment looks pale, feels depressed, and is partially immobilized. In advanced scurvy there are open, suppurating wounds and loss of teeth and, eventually, death. The human body can store only a certain amount of vitamin C, and so the body soon depletes itself if fresh supplies are not consumed.
It has been shown that smokers who have diets poor in vitamin C are at a higher risk of lung-borne diseases than those smokers who have higher concentrations of Vitamin C in the blood.
History of human understanding
The need to include fresh plant food or raw animal flesh in the diet to prevent disease was known from ancient times. Native peoples living in marginal areas incorporated this into their medicinal lore. For example, spruce needles were used in temperate zones in infusions, or the leaves from species of drought-resistant trees in desert areas. In 1536, the French explorer Jacques Cartier, exploring the St. Lawrence River, used the local natives' knowledge to save his men who were dying of scurvy. He boiled the needles of the arbor vitae tree to make a tea that was later shown to contain 50 mg of vitamin C per 100 grams.
Throughout history, the benefit of plant food to survive long sea voyages has been occasionally recommended by authorities. John Woodall, the first appointed surgeon to the British East India Company, recommended the preventive and curative use of lemon juice in his book "The Surgeon's Mate", in 1617. The Dutch writer, Johann Bachstrom, in 1734, gave the firm opinion that "scurvy is solely owing to a total abstinence from fresh vegetable food, and greens; which is alone the primary cause of the disease."
While the earliest documented case of scurvy was described by Hippocrates around the year 400 BC, the first attempt to give scientific basis for the cause of this disease was by a ship's surgeon in the British Royal Navy, James Lind. Scurvy was common among those with poor access to fresh fruit and vegetables, such as remote, isolated sailors and soldiers. While at sea in May 1747, Lind provided some crew members with two oranges and one lemon per day, in addition to normal rations, while others continued on cider, vinegar, sulfuric acid or seawater, along with their normal rations. In the history of science this is considered to be the first occurrence of a controlled experiment comparing results on two populations of a factor applied to one group only with all other factors the same. The results conclusively showed that citrus fruits prevented the disease. Lind published his work in 1753 in his Treatise on the Scurvy.

Lind's work was slow to be noticed, partly because he gave conflicting evidence within the book, and partly because the British admiralty saw care for the well-being of crews as a sign of weakness. In addition, fresh fruit was very expensive to keep on board, whereas boiling it down to juice allowed easy storage but destroyed the vitamin (especially if boiled in copper kettles). Ship captains assumed wrongly that Lind's suggestions didn't work because those juices failed to cure scurvy.
It was 1795 before the British navy adopted lemons or lime as standard issue at sea. Limes were more popular as they could be found in British West Indian Colonies, unlike lemons which weren't found in British Dominions, and were therefore more expensive. This practice led to the American use of the nickname "limey" to refer to the British. Captain James Cook had previously demonstrated and proven the principle of the advantages of carrying "Sour krout" on board, by taking his crews to the Hawaiian Islands and beyond without losing any of his men to scurvy. For this otherwise unheard of feat, the British Admiralty awarded him a medal.
The name "antiscorbutic" was used in the eighteenth and nineteenth centuries as general term for those foods known to prevent scurvy, even though there was no understanding of the reason for this. These foods included but were not limited to: lemons, limes, and oranges; sauerkraut, cabbage, malt, and portable soup.
In 1907, Axel Holst and Theodor Frølich, two Norwegian physicians studying beriberi contracted aboard ship's crews in the Norwegian Fishing Fleet, wanted a small test mammal to substitute for the pigeons they used. They fed guinea pigs their test diet, which had earlier produced beriberi in their pigeons, and were surprised when scurvy resulted instead. Until that time scurvy had not been observed in any organism apart from humans, and had been considered an exclusively human disease.
Discovery of ascorbic acid
In 1912, the Polish-American biochemist Casimir Funk, while researching deficiency diseases, developed the concept of vitamins to refer to the non-mineral micro-nutrients which are essential to health. The name is a portmanteau of "vital", due to the vital role they play biochemically, and "amines" because Funk thought that all these materials were chemical amines. One of the "vitamines" was thought to be the anti-scorbutic factor, long thought to be a component of most fresh plant material.
In 1928 the Arctic anthropologist Vilhjalmur Stefansson attempted to prove his theory of how the Eskimos are able to avoid scurvy with almost no plant food in their diet, despite the disease striking European Arctic explorers living on similar high-meat diets. Stefansson theorised that the natives get their vitamin C from fresh meat that is minimally cooked. Starting in February 1928, for one year he and a colleague lived on an exclusively minimally-cooked meat diet while under medical supervision; they remained healthy. (Later studies done after vitamin C could be quantified in mostly-raw traditional food diets of the Yukon, Inuit, and Métís of the Northern Canada, showed that their daily intake of vitamin C averaged between 52 and 62 mg/day, an amount approximately the dietary reference intake (DRI), even at times of the year when little plant-based food were eaten.)
From 1928 to 1933, the Hungarian research team of Joseph L Svirbely and Albert Szent-Györgyi and, independently, the American Charles Glen King, first isolated the anti-scorbutic factor, calling it "ascorbic acid" for its vitamin activity. Ascorbic acid turned out not to be an amine, or even to contain any nitrogen. For their accomplishment, Szent-Györgyi was awarded the 1937 Nobel Prize in Medicine.
Between 1933 and 1934, the British chemists Sir Walter Norman Haworth and Sir Edmund Hirst and, independently, the Polish chemist Tadeus Reichstein, succeeded in synthesizing the vitamin, making it the first to be artificially produced. This made possible the cheap mass-production of what was by then known as vitamin C. Only Haworth was awarded the 1937 Nobel Prize in Chemistry for this work, but the "Reichstein process" retained Reichstein's name.
In 1934 Hoffmann–La Roche became the first pharmaceutical company to mass-produce synthetic vitamin C, under the brand name of Redoxon.
In 1957 the American J.J. Burns showed that the reason some mammals were susceptible to scurvy was the inability of their liver to produce the active enzyme L-gulonolactone oxidase, which is the last of the chain of four enzymes which synthesize vitamin C. American biochemist Irwin Stone was the first to exploit vitamin C for its food preservative properties. He later developed the theory that humans possess a mutated form of the L-gulonolactone oxidase coding gene.
Daily requirements
The North American Dietary Reference Intake recommends 90 milligrams per day and no more than 2 grams per day (2000 milligrams per day). Other related species sharing the same inability to produce vitamin C and requiring exogenous vitamin C consume 20 to 80 times this reference intake. There is continuing debate within the orthodox scientific community over the best dose schedule (the amount and frequency of intake) of vitamin C for maintaining optimal health in humans. It is generally agreed that a balanced diet without supplementation contains enough vitamin C to prevent scurvy in an average healthy adult, while those who are pregnant, smoke tobacco, or are under stress require slightly more.
High doses (thousands of milligrams) may result in diarrhea in healthy adults. Proponents of alternative medicine (specifically orthomolecular medicine) claim the onset of diarrhea to be an indication of where the body’s true vitamin C requirement lies, though this has yet to be clinically verified.
| United States vitamin C recommendations | |
|---|---|
| Recommended Dietary Allowance (adult male) | 90 mg per day |
| Recommended Dietary Allowance (adult female) | 75 mg per day |
| Tolerable Upper Intake Level (adult male) | 2,000 mg per day |
| Tolerable Upper Intake Level (adult female) | 2,000 mg per day |
Government recommended intakes
Recommendations for vitamin C intake have been set by various national agencies:
- 40 milligrams per day: the United Kingdom's Food Standards Agency
- 45 milligrams per day: the World Health Organization
- 60 mg/day: Health Canada 2007
- 60–95 milligrams per day: United States' National Academy of Sciences
The United States defined Tolerable Upper Intake Level for a 25-year-old male is 2,000 milligrams per day.
Alternative recommendations on intakes
Some independent researchers have calculated the amount needed for an adult human to achieve similar blood serum levels as vitamin C synthesising mammals as follows:
- 400 milligrams per day: the Linus Pauling Institute.
- 500 milligrams per 12 hours: Professor Roc Ordman, from research into biological free radicals.
- 3,000 milligrams per day (or up to 300,000 mg during illness): the Vitamin C Foundation.
- 6,000–12,000 milligrams per day: Thomas E. Levy, Colorado Integrative Medical Centre.
- 6,000–18,000 milligrams per day: Linus Pauling's personal use.
Vitamin C high dose arguments
There is a strong advocacy movement for large doses of vitamin C based on some in vitro and retrospective studies , though large, randomized clinical trials are still lacking. Many pro-vitamin C organizations promote usage levels well beyond the current Dietary Reference Intake. The movement is led by scientists and doctors such as Robert Cathcart, Ewan Cameron, Steve Hickey, Irwin Stone and the twice Nobel Prize laureate Linus Pauling and Dr Matthias Rath. Pauling's 1986 book How to Live Longer and Feel Better was a bestseller and advocated taking many grams per day orally. There is some scientific literature critical of governmental agency dose recommendations. The biological halflife for vitamin C is fairly short, about 30 minutes in blood plasma, a fact which high dose advocates say that mainstream researchers have failed to take into account. Researchers at the National Institutes of Health decided upon the current RDA based upon tests conducted 12 hours (24 half lives) after consumption. Mainstream medicine remains skeptical of these claims.
Genetic rationales for high doses
Four gene products are necessary to manufacture vitamin C from glucose. The loss of activity of the gene for the last step, Pseudogene ΨGULO (GLO) the terminal enzyme responsible for manufacture of vitamin C, has occurred separately in the history of several species. The loss of this enzyme activity is responsible of inability of guinea pigs to synthesize vitamin C enzymatically, but this event happened independently of the loss in the haplorrhini suborder of primates, including humans. The remains of this non-functional gene with many mutations, is however still present in the genome of the guinea pigs and in primates, including humans. GLO activity has also been lost in all major families of bats, regardless of diet. In addition, the function of GLO appears to have been lost several times, and possibly re-acquired, in several lines of passerine birds, where ability to make vitamin C varies from species to species.
Loss of GLO activity in the primate order supposedly occurred about 63 million years ago, at about the time it split into the suborders haplorrhini (which lost the enzyme activity) and the more primitive strepsirrhini (which retained it). The haplorrhini ("simple nosed") primates, which cannot make vitamin C enzymatically, include the tarsiers and the simians (apes, monkeys and humans). The suborder strepsirrhini (bent or wet-nosed prosimians) which are still able to make vitamin C enzymatically, include lorises, galagos, pottos, and to some extent, lemurs.
Stone and Pauling calculated, based on the diet of our primate cousins (similar to what our common descendants are likely to have consumed when the gene mutated), that the optimum daily requirement of vitamin C is around 2,300 milligrams for a human requiring 2,500 kcal a day.
The established RDA has been criticized by Pauling to be one that will prevent acute scurvy, and is not necessarily the dosage for optimal health.
Therapeutic uses
Since its discovery vitamin C has been considered by some enthusiastic proponents a " universal panacea", although this led to suspicions by others of it being over-hyped. Other proponents of high dose vitamin C consider that if it is given "in the right form, with the proper technique, in frequent enough doses, in high enough doses, along with certain additional agents and for a long enough period of time," it can prevent and, in many cases, cure, a wide range of common and/or lethal diseases, notably the common cold and heart disease, although the NIH considers there to be "fair scientific evidence against this use." Some proponents issued controversial statements involving it being a cure for AIDS, bird flu, and SARS.
Probably the most controversial issue, the putative role of ascorbate in the management of AIDS, is still unresolved, more than 16 years after a study published in the Proceedings of National Academy of Sciences (USA) showing that non toxic doses of ascorbate suppress HIV replication in vitro. Other studies expanded on those results, but still, no large scale trials have yet been conducted.
In an animal model of lead intoxication, vitamin C demonstrated "protective effects" on lead-induced nerve and muscle abnormalities In smokers, blood lead levels declined by an average of 81% when supplemented with 1000 mg of vitamin C, while 200 mg were ineffective, suggesting that vitamin C supplements may be an "economical and convenient" approach to reduce lead levels in the blood. The Journal of the American Medical Association published a study which concluded, based on an analysis of blood lead levels in the subjects of the Third National Health and Nutrition Examination Survey, that the independent, inverse relationship between lead levels and vitamin C in the blood, if causal, would "have public health implications for control of lead toxicity".
Vitamin C has limited popularity as a treatment for autism spectrum symptoms. A 1993 study of 18 children with ASD found some symptoms reduced after treatment with vitamin C, but these results have not been replicated. Small clinical trials have found that vitamin C might improve the sperm count, sperm motility, and sperm morphology in infertile men, or improve immune function related to the prevention and treatment of age-associated diseases. However, to date, no large clinical trials have verified these findings.
A preliminary study published in the Annals of Surgery found that the early administration of antioxidant supplementation using α-tocopherol and ascorbic acid reduces the incidence of organ failure and shortens ICU length of stay in this cohort of critically ill surgical patients. More research on this topic is pending.
Dehydroascorbic acid, the main form of oxidized Vitamin C in the body, was shown to reduce neurological deficits and mortality following stroke, due to its ability to cross the blood-brain barrier, while "the antioxidant ascorbic acid (AA) or vitamin C does not penetrate the blood-brain barrier". In this study published by the Proceedings of the National Academy of Sciences in 2001, the authors concluded that such "a pharmacological strategy to increase cerebral levels of ascorbate in stroke has tremendous potential to represent the timely translation of basic research into a relevant therapy for thromboembolic stroke in humans". No such "relevant therapies" are available yet and no clinical trials have been planned.
In January 2007 the US Food and Drug Administration approved a Phase I toxicity trial to determine the safe dosage of intravenous vitamin C as a possible cancer treatment for "patients who have exhausted all other conventional treatment options." Additional studies over several years would be needed to demonstrate whether it is effective.
In February 2007, an uncontrolled study of 39 terminal cancer patients showed that, on subjective questionnaires, patients reported an improvement in health, cancer symptoms, and daily function after administration of high-dose intravenous vitamin C. The authors concluded that "Although there is still controversy regarding anticancer effects of vitamin C, the use of vitamin C is considered a safe and effective therapy to improve the quality of life of terminal cancer patients".
Vitamin C has been shown to lower IOP in glaucoma patients when taken in massive amounts according to the September 2007 issue of GLEAMS.
Testing for ascorbate levels in the body
Simple tests use DCPIP to measure the levels of vitamin C in the urine and in serum or blood plasma. However these reflect recent dietary intake rather than the level of vitamin C in body stores. Reverse phase high performance liquid chromatography is used for determining the storage levels of vitamin C within lymphocytes and tissue.
It has been observed that while serum or blood plasma levels follow the circadian rhythm or short term dietary changes, those within tissues themselves are more stable and give a better view of the availability of ascorbate within the organism. However, very few hospital laboratories are adequately equipped and trained to carry out such detailed analyses, and require samples to be analyzed in specialized laboratories.
Adverse effects
Common side-effects
Relatively large doses of vitamin C may cause indigestion, particularly when taken on an empty stomach.
When taken in large doses, vitamin C causes diarrhea in healthy subjects. In one trial, doses up to 6 grams of ascorbic acid were given to 29 infants, 93 children of preschool and school age, and 20 adults for more than 1400 days. With the higher doses, toxic manifestations were observed in five adults and four infants. The signs and symptoms in adults were nausea, vomiting, diarrhea, flushing of the face, headache, fatigue and disturbed sleep. The main toxic reactions in the infants were skin rashes. On the other hand, Cathcart has demonstrated that sick patients, with influenza and cancer for example, do not suffer any adverse effects whatsoever until the dosage is raised to fairly high levels such as 100 grams or higher.
Possible side-effects
As vitamin C enhances iron absorption, iron poisoning can become an issue to people with rare iron overload disorders, such as haemochromatosis. A genetic condition that results in inadequate levels of the enzyme glucose-6-phosphate dehydrogenase (G6PD), can cause sufferers to develop hemolytic anaemia after ingesting specific oxidizing substances, such as very large dosages of vitamin C.
There is a longstanding belief among the mainstream medical community that vitamin C causes kidney stones, which is based on little science. Although some individual recent studies have found a relationship there is no clear relationship between excess ascorbic acid intake and kidney stone formation.
In a study conducted on rats, during the first month of pregnancy, high doses of vitamin C may suppress the production of progesterone from the corpus luteum. Progesterone, necessary for the maintenance of a pregnancy, is produced by the corpus luteum for the first few weeks, until the placenta is developed enough to produce its own source. By blocking this function of the corpus luteum, high doses of vitamin C (1000+ mg) are theorized to induce an early miscarriage.
In a group of spontaneously aborting women at the end of the first trimester, the mean values of vitamin C were significantly higher in the aborting group. However, the authors do state: 'This could not be interpreted as an evidence of causal association.'
However, in a previous study of 79 women with threatened, previous spontaneous, or habitual abortion, Javert and Stander (1943) had 91% success with 33 patients who received vitamin C together with bioflavinoids and vitamin K (only three abortions), whereas all of the 46 patients who did not receive the vitamins aborted.
Chance of overdose
As discussed previously, vitamin C exhibits remarkably low toxicity. The LD50 (the dose that will kill 50% of a population) in rats is generally accepted to be 11.9 grams per kilogram of body weight when taken orally. The LD50 in humans remains unknown, owing to medical ethics that preclude experiments which would put patients at risk of harm. However, as with all substances tested in this way, the LD50 is taken as a guide to its toxicity in humans and no data to contradict this has been found.