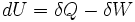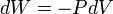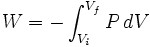Work (thermodynamics)
2008/9 Schools Wikipedia Selection. Related subjects: Physics
| Thermodynamic potentials | |
|---|---|
| Internal energy | U(S,V) |
| Helmholtz free energy | A(T,V) = U − TS |
| Enthalpy | H(S,p) = U + pV |
| Gibbs free energy | G(T,p) = U + pV − TS |
In thermodynamics, work is the quantity of energy transferred from one system to another without an accompanying transfer of entropy. It is a generalization of the concept of mechanical work in mechanics. In the SI system of measurement, work is measured in joules (symbol: J). The rate at which work is performed is power.
History
1824
Work, i.e. "weight lifted through a height", was originally defined in 1824 by Sadi Carnot in his famous paper Reflections on the Motive Power of Fire. Specifically, according to Carnot:
| “ | We use here motive power (work) to express the useful effect that a motor is capable of producing. This effect can always be likened to the elevation of a weight to a certain height. It has, as we know, as a measure, the product of the weight multiplied by the height to which it is raised. | ” |
1845
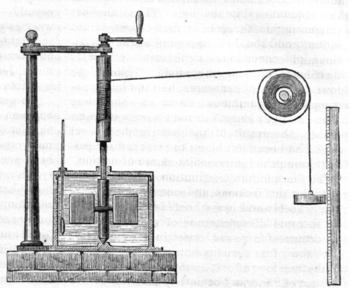
In 1845, the English physicist James Joule wrote a paper On the mechanical equivalent of heat for the British Association meeting in Cambridge. In this work, he reported his best-known experiment, in which the work released through the action of a "weight falling through a height" was used to turn a paddle-wheel in an insulated barrel of water.
In this experiment, the friction and agitation of the paddle-wheel on the body of water caused heat to be generated which, in turn, increased the temperature of water. Both the temperature change ∆T of the water and the height of the fall ∆h of the weight mg were recorded. Using these values, Joule was able to determine the mechanical equivalent of heat. Joule estimated a mechanical equivalent of heat to be 819 ft•lbf/Btu (4.41 J/cal). The modern day definitions of heat, work, temperature, and energy all have connection to this experiment.
Overview
According to the First Law of Thermodynamics, it is useful to separate changes to the internal energy of a thermodynamic system into two sorts of energy transfers. Work refers to forms of energy transfer which can be accounted for in terms of changes in the macroscopic physical variables of the system, for example energy which goes into expanding the volume of a system against an external pressure, by driving a piston-head out of a cylinder against an external force. This is in contrast to heat energy, which is carried into or out of the system in the form of transfers in the microscopic thermal motions of particles.
The concept of thermodynamic work is slightly more general than that of mechanical work because it includes other types of energy transfers as well. The electrical work required to move a charge against an external electrical field can be measured, as can the work required to move heat against a temperature gradient. An extremely important fact to understand is that thermodynamic work need not have any mechanical component to be considered such.
Mathematical definition
According to the First Law of Thermodynamics, any net increase in the internal energy U of a thermodynamic system must be fully accounted for, in terms of heat δQ entering the system minus work δW done by the system:
The letter d indicates that internal energy U is a property of the state of the system, so changes in the internal energy are exact differentials; they depend only on the original state and the final state, and not upon the path taken. In contrast, the Greek δs in this equation reflect the fact that the heat transfer and the work transfer are not properties of the final state of the system. Given only the initial state and the final state of the system, one can only say what the total change in internal energy was, not how much of the energy went out as heat, and how much as work. This can be summarized by saying that heat and work are not state functions of the system.
Pressure-volume work
Chemical thermodynamics studies PV work, which occurs when the volume of a fluid changes. PV work is represented by the following differential equation:
where:
- W = work done on the system
- P = external pressure
- V = volume
Like all work functions, PV work is path-dependent. (The path in question is a curve in the Euclidean space specified by the fluid's pressure and volume, and infinitely many such curves are possible.) From a thermodynamic perspective, this fact implies that PV work is not a state function. This means that the differential dW is an inexact differential; to be more rigorous, it should be written đW (with a line through the d).
In other words, from a mathematical point of view, đW is not an exact one-form. The line-through is merely a flag to warn us there is actually no function ( 0-form) W which is the potential of đW. If there were, indeed, this function W, we should be able to just use Stokes Theorem to evaluate this putative function, the potential of đW, at the boundary of the path, that is, the initial and final points, and therefore the work would be a state function. This impossibility is consistent with the fact that it does not make sense to refer to the work on a point in the PV diagram; work presupposes a path.
PV work is often measured in the (non-SI) units of litre-atmospheres, where 1 L·atm = 101.3 J.
Free energy and exergy
The amount of useful work which can be extracted from a thermodynamic system is discussed in the article Second Law of Thermodynamics. Under many practical situations this can be represented by the thermodynamic Availability or Exergy function. Two important cases are: in thermodynamic systems where the temperature and volume are held constant, the measure of "useful" work attainable is the Helmholtz free energy function; and in systems where the temperature and pressure are held constant, the measure of "useful" work attainable is to the Gibbs free energy.