From Wikipedia, the free encyclopedia
 |
This is a file from the Wikimedia Commons. The description on its description page there is shown below.Commons is a freely licensed media file repository. You can help.
|
 |
This is a featured picture on English Wikipedia and is considered one of the finest images.
- If you think this file should be featured on Wikimedia Commons as well, feel free to nominate it.
- If you have an image of similar quality that can be published under a suitable copyright license, be sure to upload it, tag it, and nominate it.
|
 |
Summary
| Description |
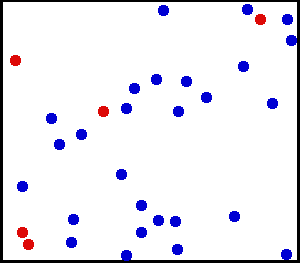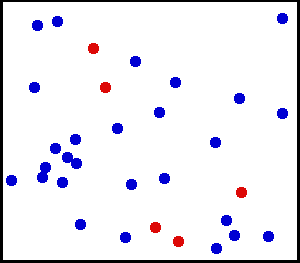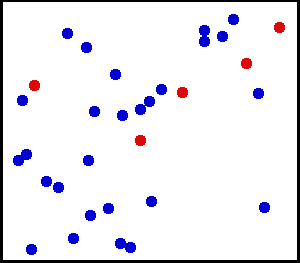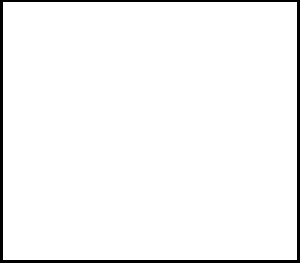
Motion of gas molecules Español: Animación mostrando la agitación térmica de un gas. Cinco partículas han sido coloreadas de rojo para facilitar el seguimiento de sus movimientos.
|
| Source |
English Wikipedia |
| Date |
August 14th 1995 |
| Author |
A.Greg, en:User:Greg L |
Permission
( Reusing this image) |
see below |
| Other versions |
Single frame (for thumbnail purposes) |
Translational motions—the randomized thermal vibrations of fundamental particles such as atoms and molecules—gives a substance its “kinetic temperature.” Here, the size of helium atoms relative to their spacing is shown to scale under 1950 atmospheres of pressure. These room-temperature atoms have a certain, average speed (slowed down here two trillion fold). At any given instant however, a particular helium atom may be moving much faster than average while another may be nearly motionless. The rebound kinetics of elastic collisions are accurately modeled here. If the velocities over time are plotted on a histogram, a Maxwell-Boltzmann distribution curve will be generated. Five atoms are colored red to facilitate following their motions.
Note that whereas the relative size, spacing, and scaled velocity of the atoms shown here accurately represent room-temperature helium atoms at a pressure of 1950 atmospheres, this is a two-dimensional scientific model; the atoms of gases in the real world aren’t constrained to moving in two dimensions in windows precisely one atom thick. If reality worked like this animation, there would be zero pressure on the two faces of the box bounding the Z-axis. The value of 1950 atmospheres is that which would be achieved if room-temperature helium atoms had the same inter-atomic separation in 3-D as they have in this 2-D animation.
Licensing
|
NOTE: "subject to disclaimers" below may not actually apply, this was tagged with {{ GFDL-user-en}}, and after May 2007, w:en:Template:GFDL-self did not require disclaimers. Please check the image description page on the English Wikipedia (or, if it has been deleted, ask an English Wikipedia administrator). See Wikipedia:GFDL standardization for details. |
Greg L at the English language Wikipedia, the copyright holder of this work, has published or hereby publishes it under the following license:
 |
Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts.
Subject to disclaimers.
Asturianu | Български | Català | Deutsch | English | Español | Français | Gaeilge | Italiano | Polski | Português | +/- |
|
|
|
Note: This tag should not be used. For images that were released on the English Wikipedia using either GFDL or GFDL-self with disclaimers, use {{ GFDL-user-en-with-disclaimers}}. For images without disclaimers please use {{ GFDL-user-en-no-disclaimers}} instead. If you are the copyright holder of files that were released on Wikipedia consider removing the disclaimers. |
File history
Click on a date/time to view the file as it appeared at that time.
|
|
Date/Time |
Dimensions |
User |
Comment |
| current |
03:31, 28 March 2008 |
300×263 (398 KB) |
Greg A L |
|
|
|
18:44, 30 October 2006 |
300×263 (398 KB) |
EdC |
|
File links
The following pages on Schools Wikipedia link to this image (list may be incomplete):