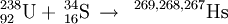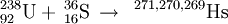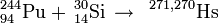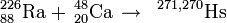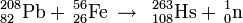Hassium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | hassium, Hs, 108 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 8, 7, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | unknown, probably silvery white or metallic gray |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [277] g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d6 7s2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 14, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | presumably a solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | unknown | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 54037-57-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hassium (pronounced /ˈhæsiəm/ or /ˈhɑːsiəm/) is a synthetic element in the periodic table that has the symbol Hs and atomic number 108.
Hassium oxidizes similarly to osmium above it, to a hassium tetroxide with a lower volatility than osmium tetroxide.
Official discovery
Hassium was first synthesized in 1984 by a German research team led by Peter Armbruster and Gottfried Münzenberg at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung) in Darmstadt. The team bombarded a lead target with iron-58 nuclei to produce 3 atoms of 265Hs in the reaction
The IUPAC/IUPAP Transfermium Working Group (TWG) recognised the GSI collaboration as official discoverers in their 1992 report.
Naming
Element 108 has historically been known as eka-osmium. During the period of controversy over the names of the elements (see element naming controversy) IUPAC adopted unniloctium (/ˌjuːnɨˈlɒktiəm/ or /ˌʌnɨˈlɒktiəm/, symbol Uno) as a temporary element name for this element.
The name hassium was proposed by the officially recognised German discoverers in 1992, derived from the Latin name for the German state of Hesse where the institute is located (L. hassia German Hessen).
In 1994 a committee of IUPAC recommended that element 108 be named hahnium (Hn).
The name hassium (Hs) was adopted internationally in 1997.
Electronic structure
Hassium has 6 full shells, 7 s+5 p+3 d+2 f=17 full subshells, and 108 orbitals:
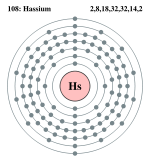
Bohr model: 2, 8, 18, 32, 32, 14, 2
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d6
Extrapolated chemical properties of eka-osmium/dvi-ruthenium
Oxidation states
Element 108 is projected to be the fifth member of the 6d series of transition metals and the heaviest member of group VIII in the Periodic Table, below iron, ruthenium and osmium. The latter two members of the group readily portray their group oxidation state of +VIII and the state becomes more stable as the group is descended. Thus hassium is expected to form a stable +VIII state. Osmium also shows stable +V, +IV and +III states with the +IV state the most stable. For ruthenium, the +VI, +V and +III states are stable with the +III state being the most stable. Hassium is therefore expected to also show other stable lower oxidation states.
Chemistry
The group VIII elements show a vary distinctive oxide chemistry which allows facile extrapolations to be made for hassium. All the lighter members have known or hypothetical tetroxides, MO4. The oxidising power decreases as one descends the group such that FeO4 is not known due to an extraordinary electron affinity which results in the formation of the well-known oxo-ion ferrate(VI), FeO42−. Ruthenium tetroxide, RuO4, formed by oxidation of ruthenium(VI) in acid, readily undergoes reduction to ruthenate(VI), RuO42−. Oxidation of ruthenium metal in air forms the dioxide, RuO2. In contrast, osmium burns to form the stable tetroxide, OsO4, which complexes with hydroxide ion to form an osmium(VIII) -ate complex, [OsO4(OH)2]2−. Therefore, eka-osmium properties for hassium should be demonstrated by the formation of a volatile tetroxide HsO4, which undergoes complexation with hydroxide to form a hassate(VII), [HsO4(OH)2]2−.
Experimental chemistry
Gas phase chemistry
Hassium is expected to have the electron configuration [Rn]5f14 6d6 7s2 and thus behave as the heavier homolog of osmium (Os). As such, it should form a volatile tetroxide, HsO4, due to the tetrahedral shape of the molecule.
The first chemistry experiments were performed using gas thermochromatography in 2001, using 172Os as a reference. During the experiment, 5 hassium atoms were detected using the reaction 248Cm(26Mg,5n)269Hs. The resulting atoms were thermalized and oxidized in a He/O2 mixture to form the oxide. The measured deposition temperature indicated that hassium(VIII) oxide is less volatile than osmium tetroxide, OsO4, and places hassium firmly in group 8.
In order to further probe the chemistry of hassium, scientists decided to assess the reaction between hassium tetroxide and sodium hydroxide to form the sodium hassate(VIII), a reaction well-known with osmium. In 2004, scientists announced that they had succeeded in carrying out the first acid-base reaction with a hassium compound.
Summary of compounds and complex ions
| Formula | Names(s) |
|---|---|
| HsO4 | hassium tetroxide; hassium(VIII) oxide |
| Na2[HsO4(OH)2] | sodium hassate(VIII); disodium dihydroxytetraoxohassate(VIII) |
History of synthesis of isotopes by cold fusion
136Xe(136Xe,xn)272−xHs
Important future experiments will involve the attempted synthesis of hassium isotopes in this symmetric reaction using the fission fragments. There are inconfirmed reports that the reaction was carried out at Dubna in 2007 and that no atoms were detected, leading to a cross section limit of 1 pb. If confirmed, this would indicate that such symmetric fusion reactions should be modelled as 'hot fusion' reactions rather than 'cold fusion' ones, as first suggested. This would indicate that such reactions will unfortunately have limited use in the synthesis of superheavy elements.
198Pt(70Zn,xn)268−xHs
This reaction was performed in May 2002 at the GSI. Unfortunately, the experiment was cut short due to a failure of the zinc-70 beam.
208Pb(58Fe,xn)266−xHs (x=1,2)
This reaction was first reported in 1978 by the team at Dubna. In a later experiment in 1984, using the rotating drum technique, they were able to detect a spontaneous fission activity assigned to 260Sg, daughter of 264Hs. In a repeat experiment in the same year, they applied the method of chemical identification of a descendant to provide support to the synthesis of element 108. They were able to detect several alpha decays of 253Es and 253Fm, descendants of 265108.
In the official discovery of the element in 1984, the team at GSI studied the reaction using the alpha decay genetic correlation method. They were able to positively identify 3 atoms of 265Hs. After an upgrade of their facilities in 1993, the team repeated the experiment in 1994 and detected 75 atoms of 265Hs and 2 atoms of 264Hs, during the measurement of a partial excitation function for the 1n neutron evaporation channel. The maximum of the 1n channel was measured as 69 pb in a further run in late 1997 in which a further 20 atoms were detected.
The discovery experiment was successfully repeated in 2002 at RIKEN (10 atoms) and in 2003 at GANIL (7 atoms).
207Pb(58Fe,xn)265−xHs (x=1)
The use of a Pb-207 target was first used in 1984 at Dubna. They were able to detect the same SF activity as observed in the Pb-208 run and once again assigned it to 260Sg, daughter of 264Hs. The team at GSI first studied the reaction in 1986 using the method of correlation of genetic alpha decays and identified a single atom of 264Hs with a cross section of 3.2 pb. The reaction was repeated in 1994 and the team were able to measure both alpha decay and spontaneous fission for 264Hs.
209Bi(55Mn,xn)264−xHs
First attempts to synthesie nuclei of element 108 were performed using this reaction by the team at Dubna in 1983. Using the rotating drum technique, they were able to detect a spontaneous fission activity assigned to 255Rf, descendant of the 263108 decay chain. Identical results were measured in a repeat run in 1984. In a subsequent experiment in 1983, they applied the method of chemical identification of a descendant to provide support to the synthesis of element 108. They were able to detect alpha decays from fermium isotopes, assigned as descendants of the decay of 262108. This reaction has not been tried since and 263Hs and 262Hs are currently inconfirmed.
History of synthesis of isotopes by hot fusion
226Ra(48Ca,xn)274−xHs
This reaction was reportedly studied in 1978 by the team at the Flerov Laboratory of Nuclear Reactions (FLNR) under the leadership of Yuri Oganessian. However, results are not available in the literature.
232Th(40Ar,xn)272−xHs
This reaction was first studied at Dubna in 1987. Detection was by spontaneous fission and no activities were found leading to a calculated cross section limit of 2 pb.
238U(34S,xn)272−xHs (x=5)
In March 1994, the team at Dubna led by the late Yuri Lazerev announced the detection of 3 atoms of 267Hs from the 5n neutron evaporation channel. The decay properties was confirmed by the team at GSI in their simultaneous study of element 110.
248Cm(26Mg,xn)274−xHs (x=3,4,5)
Most recently, a GSI-PSI collaboration has studied the nuclear reaction of curium-248 with magnesium-26 ions. Between May 2001 and August 2005, the team has studied the excitation function of the 3n, 4n, and 5n evaporation channels leading to 269Hs, 270Hs, and 271Hs. The synthesis of the important isotope 270Hs was published in December 2006 by the team of scientists from the Technical University of Munich.. It was reported that this isotope decayed by emission of an alpha-particle with an energy of 8.83 MeV and a projected half-life of ~22 s, assuming a 0+ to 0+ ground state decay to 266Sg using the Viola-Seaborg equation.
248Cm(25Mg,xn)273−xHs
This new reaction was studied at the GSI in July-August 2006 in a search for the new isotope 268Hs. They were unable to detect any atoms from neutron evaporation and calculated a cross section limit of 1 pb.
249Cf(22Ne,xn)271−xHs
The team at Dubna studied this reaction in 1983 using detection by spontaneous fission (SF). Several short SF activities were found indicating the formation of nuclei of element 108.
Chemical yields of isotopes
The tables below provides cross-sections and excitation energies for reactions producing hassium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
Cold fusion
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 58Fe | 208Pb | 266Hs | 69 pb, 13.9 MeV | 4.5 pb | |
| 58Fe | 207Pb | 265Hs | 3.2 pb |
Hot fusion
| Projectile | Target | CN | 3n | 4n | 5n |
|---|---|---|---|---|---|
| 34S | 238U | 272Hs | 2.5 pb, 50.0 MeV | ||
| 26Mg | 248Cm | 274Hs | 2.5 pb | 2.5 pb | 6.0 pb |
Isomerism in hassium isotopes
269Hs
The direct synthesis of 269Hs has resulted in three alpha lines at 9.21, 9.10, and 8.94 MeV. In the decay of 277112, only 9.21 MeV 269Hs alpha decays have been observed indicating that this decay occurs from an isomeric level. Further research is required to confirm this.
267Hs
The decay of 267Hs is known to occur by alpha decay with three alpha lines at 9.88, 9.83, and 9.75 MeV and a half-life of 52 ms. In the recent syntheses of 271m,gDs additional activities have been observed. A .94ms activity decaying by 9.83 MeV alpha emission has been observed in addition to longer lived ~.8 s and ~6.0 s activities. Each of these is currently not assigned and confirmed and further research is required to positively identify them.
265Hs
The synthesis of 265Hs has also provided evidence for two levels. The ground state decays by 10.30 MeV alpha emission with a halflife of 2.0 ms. The isomeric state is placed at 300 keV above the ground state and decays by 10.57 MeV alpha emission with a halflife of .75 ms.
Chronology of isotope discovery
| Isotope | Year discovered | Discoverer reaction |
|---|---|---|
| 264Hs | 1986 | 207Pb(58Fe,n) |
| 265Hs | 1984 | 208Pb(58Fe,n) |
| 266Hs | 2000 | 207Pb(64Ni,n) |
| 267Hs | 1995 | 238U(34S,5n) |
| 268Hs | unknown | |
| 269Hs | 1996 | 208Pb(70Zn,n) |
| 270Hs | 2004 | 248Cm(26Mg,4n) |
| 271Hs | 2004 | 248Cm(26Mg,3n) |
| 272Hs | unknown | |
| 273Hs | unknown | |
| 274Hs | unknown | |
| 275Hs | 2003 | 242Pu(48Ca,3n) |
| 276Hs | unknown | |
| 277Hs | 1999? | 244Pu(48Ca,3n) |
270Hs: prospects for a deformed doubly-magic nucleus
According to macroscopic-microscopic (MM) theory, Z=108 is a deformed proton magic number. This means that such nuclei are permanently deformed in their ground state but have high, narrow fission barriers to further deformation and hence relatively-long SF partial halflives. The SF halflives in this region are typically reduced by a factor of 109 in comparison with those in the vicinity of the spherical doubly-magic nucleus 298114, caused by an increase in the probability of barrier penetration by quantum tunnelling, due to the narrower fission barrier. In addition, N=162 has been calculated as a deformed neutron magic number and hence the nucleus 270Hs has promise as a deformed doubly-magic nucleus. Experimental data from the decay of Z=110 isotopes 271Ds and 273Ds, provides strong evidence for the magic nature of the N=162 subshell. The recent synthesis of 269Hs, 270Hs, and 271Hs also fully support the assignment of N=162 as a magic closed shell.
Evidence for the Z=108 deformed proton shell
Evidence for the effect of the Z=108 closed shell in the vicinity of the N=162 shell is limited at this moment in time. This is caused by the low production yields of the isotopes in question and thus poor statistics regarding SF partial halflives resulting from branching of the decay mode. In the case of the isotonic pair 264Hs and 262Sg (N=156 isotones), the lifetimes and decay modes do not support the stabilising effect of Z=108 but this is most likely due to a retreat from the N=162 shell. More conclusive evidence would come from the measurement of SF partial halflives for 266Hs (vs. 264Sg), 268Hs (vs. 266Sg), and especially 270Hs itself (vs 268Sg and 266Rf), although 268Sg and 268Hs are currently unknown and 266Rf has not been produced via alpha decay (which would provide TSF for this N=162 isotone). Analysis of partial SF halflives of nuclei with Z>108 (e.g 272Ds) would also help to confirm the Z=108 closed shell. It should be noted that whilst 270Hs is expected to be a doubly-magic nucleus, it is not expected to have the longest halflife in this region of the periodic table. The reason is that whilst the N=162 shell staves off fission, alpha decay will predominate. As an example, the nucleus 268Sg (Z=106,N=162) is calculated to have a halflife of the order of two hours. However, recent data from the decay of 264Sg (TSF = 70 ms) and 266Sg (TSF = 360 ms) indicate that the influence of the N=162 shell for seaborgium isotopes against fission is some 1–2 orders of magnitude overestimated, so 268Sg may in fact decay by SF will a short half life of ~5 s. The recently-synthesized nucleus 268Db (TSF = 29 h) has such a long halflife because the presence of both the odd proton and odd neutron hinder SF, relative to neighbouring even-even nuclei.
Unconfirmed isotopes
277Hs
An isotope assigned to 277Hs has been observed on two occasions decaying by SF with a long halflife of ~12 minutes. The isotope is not observed in the decay of ground state 281Ds but is observed in the decay from an nonconfirmed isomeric level. The halflife is very long for the ground state and it is possible that it belongs to an isomeric level in 277Hs. Further research is required to confirm this result.
Retracted isotopes
273Hs
The claimed synthesis of element 118 by LBNL in 1999 involved the intermediate 273Hs. This isotope was claimed to decay by 9.78 and 9.47 MeV alpha emission with a half-life of 1.2 s. The claim to discovery of 293118 was retracted and this hassium isotope is currently unknown.
Future experiments
The team at GSI has plans to further study the synthesis of hassium isotopes by hot fusion. During 2007–2008, they hope to study the reaction
This will give them experience at producing intense 34S beams before continuing with a measurement of the excitation function for the 3n, 4n, and 5n channels in the reaction using the expensive 36S isotope
In a continuation of studies towards the synthesis of 270Hs, two further reactions are to be studied in 2008 by the TUM and the FLNR.
The team at the Technische Universitaet Mainz (TUM) are planning to study the electrodeposition of hassium atoms using the new reaction:
In addition, scientists at the FLNR will reattempt the reaction:
The FLNR is currently the only facility which is able to utilise the intensively radioactive Ra-226 target and will help to complete a survey of production methods for the 274Hs compound nucleus, which will provide useful information on the mechanism of hot fusion.
As part of their continued program on the study of the effect of isospin on the yield of evaporation residues in cold fusion reactions, the team at LBNL are planning to study the following reaction in 2008 with the search for a new isotope 263Hs
Eka-osmium
Eka-osmium was a temporary name used to refer to the element that goes under osmium in the periodic table. The name "eka" was used in the same way as in Mendeleev's predicted elements. During the first half of the 20th century, eka-osmium referred to plutonium, because the actinide concept, which postulates the actinides form an inner transition series similar to the lanthanides, had not been proposed yet. Once the actinide concept became widely accepted, eka-osmium started to refer to element 108, now called Hassium, which was discovered in 1984.
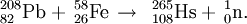
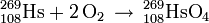
![\mathrm{HsO}_4 + 2 \,\mathrm{NaOH} \, \to \mathrm{Na}_2[\mathrm{HsO}_4(\mathrm{OH}_2)]](../../images/647/64709.png)