Polystyrene
2008/9 Schools Wikipedia Selection. Related subjects: Chemical compounds; Materials science
| Polystyrene | |
|---|---|
| Density | 1050 kg/m³ |
| Density of EPS | 25-200 kg/m³ |
| Specific Gravity | 1.05 |
| Electrical conductivity (s) | 10-16 S/ m |
| Thermal conductivity (k) | 0.08 W/(m·K) |
| Young's modulus (E) | 3000-3600 M Pa |
| Tensile strength (st) | 46–60 M Pa |
| Elongation at break | 3–4% |
| Notch test | 2–5 kJ/ m² |
| Glass temperature | 95 ° C |
| Melting point | 240 ° C |
| Vicat B | 90 ° C |
| Heat transfer coefficient (Q) | 0.17 W/( m2 K) |
| Linear expansion coefficient (a) | 8 10-5 / K |
| Specific heat (c) | 1.3 kJ/(kg·K) |
| Water absorption (ASTM) | 0.03–0.1 |
| Decomposition | X years, still decaying |
Polystyrene IPA: /ˌpɒliˈstaɪriːn/ ( IUPAC Polyphenylethene) is an aromatic polymer made from the aromatic monomer styrene, a liquid hydrocarbon that is commercially manufactured from petroleum by the chemical industry. Polystyrene is a thermoplastic substance, normally existing in solid state at room temperature, but melting if heated (for molding or extrusion), and becoming solid again when cooling off.
Pure solid polystyrene is a colorless, hard plastic with limited flexibility. It can be cast into molds with fine detail. Polystyrene can be transparent or can be made to take on various colours. It is economical and is used for producing plastic model assembly kits, license plate frames, plastic cutlery, CD "jewel" cases, and many other objects where a fairly rigid, economical plastic is desired.
History
Polystyrene was discovered in 1839 by Eduard Simon, an apothecary in Berlin. From storax, the resin of Liquidambar orientalis, he distilled an oily substance, a monomer which he named styrol. Several days later Simon found that the styrol had thickened, presumably from oxidation, into a jelly he dubbed styrol oxide ("Styroloxyd"). By 1845 English chemist John Blyth and German chemist August Wilhelm von Hofmann showed that the same transformation of styrol took place in the absence of oxygen. They called their substance metastyrol. Analysis later showed that it was chemically identical to Styroloxyd. In 1866 Marcelin Berthelot correctly identified the formation of metastyrol from styrol as a polymerization process. About 80 years went by before it was realized that heating of styrol starts a chain reaction which produces macromolecules, following the thesis of German organic chemist Hermann Staudinger (1881–1965). This eventually led to the substance receiving its present name, polystyrene. The I. G. Farben company began manufacturing polystyrene in Ludwigshafen, Germany, about 1931, hoping it would be a suitable replacement for die cast zinc in many applications. Success was achieved when they developed a reactor vessel that extruded polystyrene through a heated tube and cutter, producing polystyrene in pellet form.
Structure
The chemical makeup of polystyrene is a long chain hydrocarbon with every other carbon connected to a Phenyl group (the name given to the aromatic ring benzene, when bonded to complex carbon substituents).
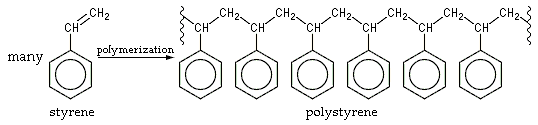
A 3-D model would show that each of the chiral backbone carbons lies at the centre of a tetrahedron, with its 4 bonds pointing toward the vertices. Say the -C-C- bonds are rotated so that the backbone chain lies entirely in the plane of the diagram. From this flat schematic, it is not evident which of the phenyl (benzene) groups are angled toward us from the plane of the diagram, and which ones are angled away. The isomer where all of them are on the same side is called isotactic polystyrene, which is not produced commercially. Ordinary atactic polystyrene has these large phenyl groups randomly distributed on both sides of the chain. This random positioning prevents the chains from ever aligning with sufficient regularity to achieve any crystallinity, so the plastic has no melting temperature, Tm. But metallocene-catalyzed polymerization can produce an ordered syndiotactic polystyrene with the phenyl groups on alternating sides. This form is highly crystalline with a Tm of 270 °C.
Solid foam
Polystyrene's most common use is as expanded polystyrene (EPS). Expanded polystyrene is produced from a mixture of about 90-95% polystyrene and 5-10% gaseous blowing agent, most commonly pentane or carbon dioxide. The solid plastic is expanded into a foam through the use of heat, usually steam.
Extruded polystyrene (XPS), which is different from expanded polystyrene (EPS), is commonly known by the trade name Styrofoam. The voids filled with trapped air give it low thermal conductivity. This makes it ideal as a construction material and it is therefore sometimes used in structural insulated panel building systems. It is also used as insulation in building structures, as molded packing material for cushioning fragile equipment inside boxes, as packing "peanuts", as non-weight-bearing architectural structures (such as pillars), and also in crafts and model building, particularly architectural models. Foamed between two sheets of paper, it makes a more-uniform substitute for corrugated cardboard, tradenamed Foamcore. A more unexpected use for the material is as a lightweight fill for embankments in the civil engineering industry .
Expanded polystyrene used to contain CFCs, but other, more environmentally-safe blowing agents are now used. Because it is an aromatic hydrocarbon, it burns with an orange-yellow flame, giving off soot, as opposed to non-aromatic hydrocarbon polymers such as polyethylene, which burn with a light yellow flame (often with a blue tinge) and no soot.
Production methods include sheet stamping (PS) and injection molding (both PS and HIPS).
The density of expanded polystyrene varies greatly from around 25 kg/m³ to 200 kg/m³ depending on how much gas was admixed to create the foam. A density of 200 kg/m³ is typical for the expanded polystyrene used in surfboards.
Standard markings
The resin identification code symbol for polystyrene, developed by the Society of the Plastics Industry so that items can be labeled for easy recycling, is ![]() . However, the majority of polystyrene products are currently not recycled because of a lack of suitable recycling facilities. Furthermore, when it is "recycled," it is not a closed loop — polystyrene cups and other packaging materials are usually recycled into fillers in other plastics, or other items that cannot themselves be recycled and are thrown away.
. However, the majority of polystyrene products are currently not recycled because of a lack of suitable recycling facilities. Furthermore, when it is "recycled," it is not a closed loop — polystyrene cups and other packaging materials are usually recycled into fillers in other plastics, or other items that cannot themselves be recycled and are thrown away.
Copolymers
Pure polystyrene is brittle, but hard enough that a fairly high-performance product can be made by giving it some of the properties of a stretchier material, such as polybutadiene rubber. The two such materials can never normally be mixed because of the amplified effect of intermolecular forces on polymer insolubility (see plastic recycling), but if polybutadiene is added during polymerization it can become chemically bonded to the polystyrene, forming a graft copolymer which helps to incorporate normal polybutadiene into the final mix, resulting in high-impact polystyrene or HIPS, often called "high-impact plastic" in advertisements. One commercial name for HIPS is Bextrene. Common applications include use in toys and product casings. HIPS is usually injection molded in production. Autoclaving polystyrene can compress and harden the material.
Acrylonitrile butadiene styrene or ABS plastic is similar to HIPS: a copolymer of acrylonitrile and styrene, toughened with polybutadiene. Most electronics cases are made of this form of polystyrene, as are many sewer pipes. ABS pipes may become brittle over time. SAN is a copolymer of styrene with acrylonitrile.
Styrene can be copolymerized with other monomers; for example, divinylbenzene for cross-linking the polystyrene chains.
Cutting and shaping
Expanded polystyrene is very easily cut with a hot-wire foam cutter, which is easily made by a heated taut length of wire, usually nichrome because of nichrome's resistance to oxidation at high temperatures and its suitable electrical conductivity. The hot wire foam cutter works by heating the wire to the point where it can vaporize foam immediately adjacent to it. The foam gets vaporized before actually touching the heated wire, which yields exceptionally smooth cuts.
Polystyrene, shaped and cut with hot wire foam cutters, is used in architecture models, actual signage, amusement parks, movie sets, airplane construction, and much more. Such cutters may cost just a few dollars (for a completely manual cutter) to tens of thousands of dollars for large CNC machines that can be used in high-volume industrial production.
Polystyrene can also be cut with a traditional cutter. In order to do this without ruining the sides of the blade one must first dip the blade in water and cut with the blade at an angle of about 30º. The procedure has to be repeated multiple times for best results.
Polystyrene can also be cut on 3 and 5-axis routers, enabling large-scale prototyping and model-making. Special polystyrene cutters are available that look more like large cylindrical rasps.
Use in biology
Petri dishes and other containers such as test tubes, made of polystyrene, play an important role in biomedical research and science. For these uses, articles are almost always made by injection molding, and often sterilized post molding, either by irradiation or treatment with ethylene oxide. Post mold surface modification, usually with oxygen rich plasmas, is often done to introduce polar groups. Much of modern biomedical research relies on the use of such products; they therefore play a critical role in pharmaceutical research. Major manufacturers include Corning Incorporated/Costar, Nalgene/Nunc, Greiner and BD/Falcon. The web sites of these companies contain a wealth of information.
Finishing
In the United States, environmental protection regulations prohibit the use of solvents on polystyrene (which would dissolve the polystyrene and de-foam most of foams anyway).
Some acceptable finishing materials are
- Water-based paint ( artists have created paintings on polystyrene with gouache)
- Mortar or acrylic/cement render, often used in the building industry as a weather-hard overcoat that hides the foam completely after finishing the objects.
- Cotton wool or other fabrics used in conjunction with a stapling implement.
Dangers and fire hazard
The health effects caused by consuming polystyrene when it migrates from food containers (primarily from a leaching caused by heat exchange) into food is under serious investigation. Benzene, a material used in the production of polystyrene, is a known human carcinogen. Moreover, butadiene and styrene (in ABS), when combined, become benzene-like in both form and function.
The EPA claims
"Acute (short-term) exposure to styrene in humans results in mucous membrane and eye irritation, and gastrointestinal effects. Chronic (long-term) exposure to styrene in humans results in effects on the central nervous system (CNS), such as headache, fatigue, weakness, and depression, CSN dysfunction, hearing loss, and peripheral neuropathy. Human studies are inconclusive on the reproductive and developmental effects of styrene; several studies did not report an increase in developmental effects in women who worked in the plastics industry, while an increased frequency of spontaneous abortions and decreased frequency of births were reported in another study. Several epidemiologic studies suggest there may be an association between styrene exposure and an increased risk of leukemia and lymphoma. However, the evidence is inconclusive due to confounding factors. EPA has not given a formal carcinogen classification to styrene."
Polystyrene is classified according to DIN4102 as a "B3" product, meaning highly flammable or "easily ignited". Consequently, though it is an efficient insulator at low temperatures, it is prohibited from being used in any exposed installations in building construction as long the material is not flame retarded e.g. with hexabromocyclododecane. It must be concealed behind drywall, sheet metal or concrete. Foamed plastic materials have been accidentally ignited and caused huge fires and losses. Examples include the Düsseldorf International Airport, the Channel tunnel, where it was inside a railcar and caught on fire, and the Browns Ferry Nuclear Power Plant, where fire reached through a fire retardant, reached the foamed plastic underneath, inside a firestop that had not been tested and certified in accordance with the final installation.
In addition to fire hazard, substances that contain acetone (such as most aerosol paint sprays), and cyanoacrylate glues can dissolve polystyrene.
Environmental concerns and bans
Expanded polystyrene is not easily recyclable because of its light weight and low scrap value. It is generally not accepted in curbside programs. Expanded polystyrene foam takes a very long time to decompose in the environment and has been documented to cause starvation in birds and other marine wildlife. According to the California Coastal Commission, it is a principal component of marine debris. A CIWMB ( California Integrated Waste Management Board) Report finds that “in the categories of energy consumption, greenhouse gas effect, and total environmental effect, EPS’s environmental impacts were second highest, behind aluminium.” Restricting the use of foamed polystyrene takeout food packaging is a priority of many solid waste environmentalist organizations, like Californians Against Waste.
The city of Berkeley, California was one of the first cities in the world to ban polystyrene food packaging (called Styrofoam in the media announcements). It was also banned in Portland, OR, and Suffolk County, NY in 1990. Now, over 20 US cities have banned polystyrene food packaging, including Oakland, CA on Jan 1st 2007. San Francisco introduced a ban on the packaging on June 1st 2007:
"This is a long time coming," Peskin said Monday. "Polystyrene foam products rely on nonrenewable sources for production, are nearly indestructible and leave a legacy of pollution on our urban and natural environments. If McDonald's could see the light and phase out polystyrene foam more than a decade ago, it's about time San Francisco got with the program." Board of Supervisors President, Aaron Peskin
The overall benefits of the ban in Portland have been questioned , as have the general environmental concepts of the use of paper versus polystyrene.
A campaign to achieve the first ban of polystyrene foam from the food & beverage industry in Canada has been launched in Toronto as of January 2007, by local non-profit organization NaturoPack.
Other cities that have banned expanded polystyrene include Portland, Oakland, and Santa Monica. Both the California and New York legislatures are currently considering bills which would effectively ban expanded polystyrene in all takeout food packaging state-wide..
Explosives
Polystyrene is used in some polymer-bonded explosives:
| Name | Explosive Ingredients | Binder Ingredients | Usage |
|---|---|---|---|
| PBX-9205 | RDX 92% | Polystyrene 6%; DOP 2% | |
| PBX-9007 | RDX 90% | Polystyrene 9.1%; DOP 0.5%; resin 0.4% |
It is also a component of Napalm and a component of most designs of hydrogen bombs.
Cleaning
Polystyrene can be dishwashed at 70 °C without deformation since it has a glass transition temperature of 95 °C



