Prion
2008/9 Schools Wikipedia Selection. Related subjects: Health and medicine
| Prion Diseases (TSEs) Classification and external resources |
|
| ICD- 10 | A81 |
|---|---|
| ICD- 9 | 046 |
A prion (IPA: /ˈpriːɒn/ listen ) — combination of the first two syllables of the words proteinaceous and infectious (-on by analogy to virion) — is a poorly-understood hypothetical infectious agent that, according to the "protein only" hypothesis, is composed entirely of proteins. Prions are thought to cause a number of diseases in a variety of mammals, including bovine spongiform encephalopathy (BSE, also known as "mad cow disease") in cattle and Creutzfeldt-Jakob disease (CJD) in humans. All thus-far hypothesized prion diseases affect the structure of the brain or other neural tissue, and all are currently untreatable and are always fatal. In general usage, prion can refer to both the theoretical unit of infection or the specific protein (e.g. PrP) that is thought to be the infective agent, whether or not it is in an infective state.
Prions are hypothesized to infect and propagate by refolding abnormally into a structure which is able to convert normal molecules of the protein into the abnormally structured form. All known prions induce the formation of an amyloid fold, in which the protein polymerises into an aggregate consisting of tightly packed beta sheets. This altered structure is extremely stable and accumulates in infected tissue, causing cell death and tissue damage. This stability means that prions are resistant to denaturation by chemical and physical agents, making disposal and containment of these particles difficult.
Proteins showing prion-type behaviour are also found in some fungi and this has been quite important in helping to understand mammalian prions. However, fungal prions do not appear to cause disease in their hosts and may even confer an evolutionary advantage through a form of protein-based inheritance.
Discovery
The radiation biologist Tikvah Alper and the mathematician John Stanley Griffith developed the hypothesis during the 1960s that some transmissible spongiform encephalopathies are caused by an infectious agent consisting solely of proteins. This theory was developed to explain the discovery that the mysterious infectious agent causing the diseases scrapie and Creutzfeldt-Jakob Disease resisted ultraviolet radiation (UV radiation causes direct DNA damage by exciting individual molecules in the DNA polymer, which causes errors to be introduced into base pair sequence). Sir Francis Crick recognized the potential importance of the Griffith protein-only hypothesis for scrapie propagation in the second edition of his famous " Central dogma of molecular biology". While asserting that the flow of sequence information from protein to protein, or from protein to RNA and DNA was "precluded" by this dogma, he noted that Griffith's hypothesis was a potential contradiction to this dogma (although it was not so promoted by Griffith). Since the revised "dogma" was formulated, in part, to accommodate the then-recent discovery of reverse transcription by Howard Temin and David Baltimore (who won the Nobel Prize in 1975), proof of the protein-only hypothesis might be seen as a "sure bet" for a future Nobel Prize.
Stanley B. Prusiner of the University of California, San Francisco announced in 1982 that his team had purified the hypothetical infectious prion, and that the infectious agent consisted mainly of a specific protein - though they did not manage to satisfactorily isolate the protein until two years after Prusiner's announcement. Prusiner coined the word "prion" as a name for the infectious agent, by combining the first two syllables of the words proteinaceous and infectious (-on by analogy to virion). While the infectious agent was named a prion, the specific protein that the prion was made of was named PrP, an abbreviation for "protease resistant protein". Prusiner was awarded the Nobel Prize in Physiology or Medicine in 1997 for his research into prions.
Structure
Isoforms
The protein that prions are made of is found throughout the body, even in healthy people and animals. However, the prion protein found in infectious material has a different folding pattern and is resistant to proteases, the enzymes in the body that can normally break down proteins. The normal form of the protein is called PrPC, while the infectious form is called PrPSc — the C refers to 'cellular' or 'common' PrP, while the Sc refers to ' scrapie', a prion disease occurring in sheep. While PrPC is structurally well-defined, PrPSc is certainly polydisperse and defined at a relatively poor level. PrP can be induced to fold into other more-or-less well-defined isoforms in vitro, and their relationship to the form(s) that are pathogenic in vivo is not yet clear.
PrPC
PrPC is a normal protein found on the membranes of cells. It has 209 amino acids (in humans), one disulfide bond, a molecular weight of 35-36kDa and a mainly alpha-helical structure. Several topological forms exist; one cell surface form anchored via glycolipid and two transmembrane forms. Its function has not been fully resolved. PrPC binds copper (II) ions with high affinity. The significance of this is not clear, but it presumably relates to PrP structure or function. PrPC is readily digested by proteinase K and can be liberated from the cell surface in vitro by the enzyme phosphoinositide phospholipase C (PI-PLC), which cleaves the glycophosphatidylinositol (GPI) glycolipid anchor.
PrPSc
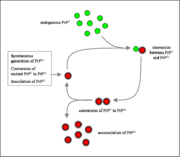
The infectious isoform of PrPC, known as PrPSc, is able to convert normal PrPC proteins into the infectious isoform by changing their conformation. Although the exact 3D structure of PrPSc is not known, there is increased β-sheet content in the diseased form of the molecule, replacing normal areas of α-helix. Aggregations of these abnormal isoforms may form highly structured amyloid fibers. The end of a fiber acts as a template for the free protein molecules, causing the fiber to grow. Small differences in the amino acid sequence of prion-forming regions lead to distinct structural features on the surface of prion fibers. As a result, only free protein molecules that are identical in amino acid sequence to the prion protein can be recruited into the growing fibre.
Function
It has now been conclusively proven that the prion protein's normal cellular role is as a copper dependent antioxidant. While a small number of researchers in the field pursue other possibilities, the majority of evidence from many researcher supports this finding.
PrP and long-term memory
There is evidence that PrP may have a normal function in maintenance of long term memory. Maglio and colleagues have shown that mice without the genes for normal cellular PrP protein have altered hippocampal LTP.
PrP and stem cell renewal
A 2006 article from the Whitehead Institute for Biomedical Research indicates that PrP expression on stem cells is necessary for an organism's self-renewal of bone marrow. The study showed that all long-term hematopoietic stem cells expressed PrP on their cell membrane and that hematopoietic tissues with such PrP-null stem cells exhibited increased sensitivity to cell depletion.
Prion disease
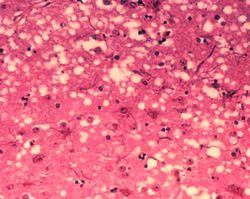
Prions cause neurodegenerative disease by aggregating extracellularly within the central nervous system to form plaques known as amyloids, which disrupt the normal tissue structure. This disruption is characterized by "holes" in the tissue with resultant spongy architecture due to the vacuole formation in the neurons. Other histological changes include astrogliosis and the absence of an inflammatory reaction. While the incubation period for prion diseases is generally quite long, once symptoms appear the disease progresses rapidly, leading to brain damage and death. Neurodegenerative symptoms can include convulsions, dementia, ataxia (balance and coordination dysfunction), and behavioural or personality changes.
All known prion diseases, collectively called transmissible spongiform encephalopathies (TSEs), are untreatable and fatal. However, a vaccine has been developed in mice that may provide insight into providing a vaccine in humans to resist prion infections. Additionally, in 2006 scientists announced that they had genetically engineered cattle lacking a necessary gene for prion production - thus theoretically making them immune to BSE, building on research indicating that mice lacking normally-occurring prion protein are resistant to infection by scrapie prion protein.
Many different mammalian species can be affected by prion diseases, as the prion protein (PrP) is very similar in all mammals. Due to small differences in PrP between different species, it is unusual for a prion disease to be transmitted from one species to another. However, the human prion disease variant Creutzfeldt-Jakob disease is believed to be caused by a prion which typically infects cattle and is transmitted through infected meat.
Some researchers have suggested that metal ion interactions with prion proteins might be relevant to the progression of prion-mediated disease, based on epidemiological studies of clusters of prion disease in locales with low soil concentrations of copper.
The following diseases are believed to be caused by prions.
- In animals:
- Scrapie in sheep and goats
- Bovine spongiform encephalopathy (BSE) in cattle (known as mad cow disease)
- Transmissible mink encephalopathy (TME) in mink
- Chronic wasting disease (CWD) in elk and mule deer
- Feline spongiform encephalopathy in cats
- Exotic ungulate encephalopathy (EUE) in nyala, oryx and greater kudu
- Spongiform encephalopathy of the ostrich
- In humans:
- Creutzfeldt-Jakob disease (CJD) and its varieties: iatrogenic Creutzfeldt-Jakob disease (iCJD), variant Creutzfeldt-Jakob disease (vCJD), familial Creutzfeldt-Jakob disease (fCJD), and sporadic Creutzfeldt-Jakob disease (sCJD)
- Gerstmann-Sträussler-Scheinker syndrome (GSS)
- Fatal familial insomnia (sFI)
- Kuru
Transmission
Although the identity and general properties of prions are now well understood, the mechanism of prion infection and propagation remains mysterious. It is often assumed that the diseased form directly interacts with the normal form to make it rearrange its structure. One idea, the "Protein X" hypothesis, is that an as-yet unidentified cellular protein (Protein X) enables the conversion of PrPC to PrPSc by bringing a molecule of each of the two together into a complex.
Current research suggests that the primary method of infection in animals is through ingestion. It is thought that prions may be deposited in the environment through the remains of dead animals and via urine, saliva, and other body fluids. They may then linger in the soil by binding to clay and other minerals.
Sterilization
Infectious particles possessing nucleic acid are dependent upon it to direct their continued replication. Prions however, are infectious by their effect on normal versions of the protein. Therefore, sterilizing prions involves the denaturation of the protein to a state where the molecule is no longer able to induce the abnormal folding of normal proteins. However, prions are generally quite resistant to denaturation by proteases, heat, radiation, and formalin treatments, although their infectivity can be reduced by such treatments.
Prion-specific methods
Prions can be denatured by subjecting them to a temperatures of 134 degrees Celsius (274 degrees Fahrenheit) for 18 minutes in a pressurised steam autoclave. Ozone sterilization is currently being studied as a potential method for prion deactivation. Renaturation of a completely denatured prion to infectious status has not yet been achieved, however partially denatured prions can be renatured to an infective status under certain artificial conditions.
The World Health Organization recommends any of the following three procedures for the sterilization of all heat-resistant surgical instruments that are potentially contaminated with prions:
- Immerse in a pan containing 1N NaOH and heat in a gravity-displacement autoclave at 121°C for 30 minutes; clean; rinse in water; and then subject to routine sterilization.
- Immerse in 1N NaOH or sodium hypochlorite (20,000 parts per million available chlorine) for 1 hour; transfer instruments to water; heat in a gravity-displacement autoclave at 121°C for 1 hour; clean; and then subject to routine sterilization
- Immerse in 1N NaOH or sodium hypochlorite (20,000 parts per million available chlorine) for 1 hour; remove and rinse in water, then transfer to an open pan and heat in a gravity-displacement (121°C) or in a porous-load (134°C) autoclave for 1 hour; clean; and then subject to routine sterilization '
Generic methods
One method that will decompose any organic material to its basic constituents uses cold (non-equilibrium) oxygen ion plasmas. [This converts the organic materials into carbon dioxide, water, nitrogen gas, nitrogen oxides, phosphorous oxides, sulfur dioxide, etc.] Even basal graphite can be converted to carbon dioxide using this method. Another way uses chromerge (Cr2O6) in concentrated sulfuric acid. This is a common method to clean glassware used in organic and analytical chemistry.
Another method for decomposing and disposing of any organic compound is burning it at high temperatures in an oxygen-rich atmosphere. This method is used for the disposal of deadly chemical weapons such as nerve gasses and mustard gas. This reduces it all to simple gaseous compounds, including water vapor, that are safe to release into the environment.
Debate
Whether prions are the agent which causes disease or merely a symptom caused by a different agent is still under debate. The following sections describe several contending hypotheses.
Protein-only hypothesis
Prior to the discovery of prions, it was thought that all pathogens used nucleic acids to direct their replication. The "protein-only hypothesis" states that a protein structure can replicate without the use of nucleic acid. This was initially controversial as it contradicts the so-called " central dogma of molecular biology," which describes nucleic acid as the central form of replicative information.
Evidence in favour of a protein-only hypothesis include:
- No virus particles, bacteria, or fungi have been conclusively associated with prion diseases
- No nucleic acid has been conclusively associated with infectivity; agent is resistant to degradation by nucleases
- No immune response to infection
- PrPSc experimentally transmitted between one species and another results in PrPSc with the amino-acid sequence of the recipient species, suggesting that replication of the donor agent does not occur
- Level of infectivity is associated with levels of PrPSc
- PrPSc and PrPC do not differ in amino-acid sequence, therefore a PrPSc-specific nucleic acid is a redundant concept
- Familial prion disease occurs in families with a mutation in the PrP gene, and mice with PrP mutations develop prion disease despite controlled conditions where transmission is prevented
Multi-component hypothesis
In 2007, biochemist Surachai Supattapone and his colleagues at Dartmouth College produced purified infectious prions de novo from defined components (PrPC, co-purified lipids, and a synthetic polyanionic molecule) . These researchers also showed that the polyanionic molecule required for prion formation was selectively incorporated into high-affinity complexes with PrP molecules, leading them to hypothesize that infectious prions may be composed of multiple host components, including PrP, lipid, and polyanionic molecules, rather than PrPSc alone .
Viral hypothesis
The protein-only hypothesis has been criticised by those who feel that the simplest explanation of the evidence to date is viral. For more than a decade, Yale University neuropathologist Laura Manuelidis has been proposing that prion diseases are caused instead by an unidentified "slow" virus. In January 2007, she and her colleagues published an article in the Proceedings of the National Academy of Science reporting to have found the virus in 10%, or less, of their scrapie-infected cells in culture.
The virion hypothesis states that TSEs are caused by a replicable informational molecule (which is likely to be a nucleic acid) bound to PrP. Many TSEs, including scrapie and BSE, show strains with specific and distinct biological properties, a feature which supporters of the virion hypothesis feel is not explained by prions. The presence of a nucleic acid bound to the protein would explain the strains observed. It has also been shown that TSEs including BSE retain their host-specific properties after passage through many different species.
Evidence in favour of a viral hypothesis include:
- No bacteria or other living organisms have been found in prion-affected organisms.
- Differences in prion infectivity, incubation, symptomology and progression among species resembles the "strain variation" seen between viruses, especially RNA viruses
- The long incubation and rapid onset of symptoms resembles some viral infections, such as HIV-induced AIDS
- A number of other properties may match the virion hypothesis more closely than the prion hypothesis, including the size of TSE agents (on which there are conflicting findings), noninfectivity induced by the disruption of what may be the agent's nucleic acid-protein structure, route of dissemination in the body (if by white blood cells, as concluded by some studies), and capacities of TSE agents similar to viral interference.
- Viral-like particles that do not appear to be composed of PrP have been found in some of the cells of scrapie- or CJD-infected cell lines.
Heavy metal poisoning hypothesis
Mark Purdey and Dr. David R. Brown have suggested that common prion is a beneficial molecule when bound to copper ions and that loss of this activity could cause disease. They have hypothesised that abnormal amounts of copper and manganese in the environment or animal feed could precipitate this.
Evidence favouring a pollutant cause:
- Manganese present increases the percentage of helical protein, while Copper decreases it.
- Alzheimer's disease has similar symptoms, and has been attributed to excessive Aluminium at various times.
- Copper deficiency and Manganese proficiency have been found in the environment of affected cattle.
- Sporadic occurrences of diseased prion rule out genetics.
Prions in yeast and other fungi
Prion proteins were discovered in the yeast Saccharomyces cerevisiae by Reed Wickner in the early 1990's. Subsequently, a prion has also been found in the fungus Podospora anserina. These prions behave similarly to PrP, but are generally non-toxic to their hosts. Susan Lindquist's group at the Whitehead Institute has argued that some of the fungal prions are not associated with any disease state, but may have a useful role; however, researchers at the NIH have also provided strong arguments demonstrating that fungal prions should be considered a diseased state. Research into fungal prions has given strong support to the protein-only hypothesis for mammalian prions, since it has been demonstrated that purified protein extracted from cells with the prion state can convert the normal form of the protein into the infectious form in vitro, and in the process, preserve the information corresponding to different strains of the prion state. It has also shed some light on prion domains, which are regions in a protein that promote the conversion into a prion. Fungal prions have helped to suggest mechanisms of conversion that may apply to all prions.