Ultraviolet
2008/9 Schools Wikipedia Selection. Related subjects: Physics

Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays. It is named because the spectrum consists of refrangible electromagnetic waves with frequencies higher than those that humans identify as the colour violet.
UV light is typically found as part of the radiation received by the Earth from the Sun. Most humans are aware of the effects of UV through the painful condition of sunburn. The UV spectrum has many other effects, including both beneficial and damaging changes to human health.
Discovery
The discovery of UV radiation was intimately associated with the observation that silver salts darken when exposed to sunlight. In 1801 the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum were especially effective at darkening silver chloride-soaked paper. He called them "de-oxidizing rays" to emphasize their chemical reactivity and to distinguish them from "heat rays" at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favour of ultraviolet and infrared radiation, respectively.
Origin of term
The name means "beyond violet" (from Latin ultra, "beyond"), violet being the colour of the shortest wavelengths of visible light. UV light has a shorter wavelength than that of violet light.
Subtypes
The electromagnetic spectrum of ultraviolet light can be subdivided in a number of ways. The draft ISO standard on determining solar irradiances (ISO-DIS-21348) describes the following ranges:
| Name | Abbreviation | Wavelength range in nanometers | Energy per photon |
|---|---|---|---|
| Ultraviolet A, long wave, or black light | UVA | 400 nm – 315 nm | 3.10 – 3.94 eV |
| Near | NUV | 400 nm – 300 nm | 3.10 – 4.13 eV |
| Ultraviolet B or medium wave | UVB | 315 nm – 280 nm | 3.94 – 4.43 eV |
| Middle | MUV | 300 nm – 200 nm | 4.13 – 6.20 eV |
| Ultraviolet C, short wave, or germicidal | UVC | 280 nm – 100 nm | 4.43 – 12.4 eV |
| Far | FUV | 200 nm – 122 nm | 6.20 – 10.2 eV |
| Vacuum | VUV | 200 nm – 10 nm | 6.20 – 124 eV |
| Extreme | EUV | 121 nm – 10 nm | 10.2 – 124 eV |
In photolithography, in laser technology, etc., the term deep ultraviolet or DUV refers to wavelengths below 300 nm. "Vacuum UV" is so named because it is absorbed strongly by air and is therefore used in a vacuum. In the long-wave limit of this region, roughly 150–200 nm, the principal absorber is the oxygen in air. Work in this region can be performed in an oxygen free atmosphere, pure nitrogen being commonly used, which avoids the need for a vacuum chamber.
See 1 E-7 m for a list of objects of comparable sizes.
Black light
A black light, or Wood's light, is a lamp that emits long wave UV radiation and very little visible light. Commonly these are referred to as simply a "UV light". Fluorescent black lights are typically made in the same fashion as normal fluorescent lights except that only one phosphor is used and the normally clear glass envelope of the bulb may be replaced by a deep-bluish-purple glass called Wood's glass, a nickel-oxide–doped glass, which blocks almost all visible light above 400 nanometers. The colour of such lamps is often referred to in the trade as "blacklight blue" or "BLB." This is to distinguish these lamps from "bug zapper" blacklight ("BL") lamps that don't have the blue Wood's glass. The phosphor typically used for a near 368 to 371 nanometer emission peak is either europium-doped strontium fluoroborate (SrB4O7F:Eu2+) or europium-doped strontium borate (SrB4O7:Eu2+) while the phosphor used to produce a peak around 350 to 353 nanometers is lead-doped barium silicate (BaSi2O5:Pb+). "Blacklight Blue" lamps peak at 365 nm.
While "black lights" do produce light in the UV range, their spectrum is confined to the longwave UVA region. Unlike UVB and UVC, which are responsible for the direct DNA damage that leads to skin cancer, black light is limited to lower energy, longer waves and does not cause sunburn. However, UVA is capable of causing damage to collagen fibers and destroying vitamin A in skin.
A black light may also be formed by simply using Wood's glass instead of clear glass as the envelope for a common incandescent bulb. This was the method used to create the very first black light sources. Though it remains a cheaper alternative to the fluorescent method, it is exceptionally inefficient at producing UV light (a mere few lumens per watt) owing to the black body nature of the incandescent light source. Incandescent UV bulbs, due to their inefficiency, may also become dangerously hot during use. More rarely still, high power (hundreds of watts) mercury vapor black lights can be found which use a UV emitting phosphor and an envelope of Wood's glass. These lamps are used mainly for theatrical and concert displays and also become very hot during normal use.
Some UV fluorescent bulbs specifically designed to attract insects for use in bug zappers use the same near-UV emitting phosphor as normal blacklights, but use plain glass instead of the more expensive Wood's glass. Plain glass blocks less of the visible mercury emission spectrum, making them appear light blue to the naked eye. These lamps are referred to as "blacklight" or "BL" in most lighting catalogs.
Ultraviolet light can also be generated by some light-emitting diodes.
Human health-related effects of UV radiation
Beneficial effects
The Earth's atmosphere blocks UV radiation from penetrating through the atmosphere by 98.7%. A positive effect of UVB exposure is that it induces the production of vitamin D in the skin. It has been estimated that tens of thousands of premature deaths occur in the United States annually from a range of cancers due to vitamin D deficiency. Another effect of vitamin D deficiency is osteomalacia (the adult equivalent of rickets), which can result in bone pain, difficulty in weight bearing and sometimes fractures. Other studies show most people get adequate Vitamin D through food and incidental exposure.
Many countries have fortified certain foods with Vitamin D to prevent deficiency. Eating fortified foods or taking a dietary supplement pill is usually preferred to UVB exposure, due to the increased risk of skin cancer from UV radiation.
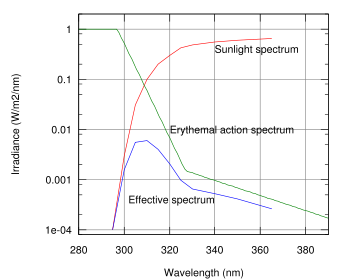
Too little UVB radiation leads to a lack of Vitamin D. Too much UVB radiation leads to direct DNA damages and sunburn. An appropriate amount of UVB (What is appropriate depends on your skin colour) leads to a limited amount of direct DNA damage. This is recognized and repaired by the body. Then the melanin production is increased which leads to a long lasting tan. This tan occurs with a 2 day lag phase after irradiation, but it is much less harmful and long lasting than the one obtained from UVA.
Ultraviolet radiation has other medical applications, in the treatment of skin conditions such as psoriasis and vitiligo. UVA radiation can be used in conjunction with psoralens ( PUVA treatment). UVB radiation is rarely used in conjunction with psoralens. In cases of psoriasis and vitiligo, UV light with wavelength of 311 nm is most effective.
Harmful effects
An overexposure to UVB radiation can cause sunburn and some forms of skin cancer. In humans, prolonged exposure to solar UV radiation may result in acute and chronic health effects on the skin, eye, and immune system. However the most deadly form - malignant melanoma - is mostly caused by the indirect DNA damage (free radicals and oxidative stress). This can be seen from the absence of a UV-signature mutation in 92% of all melanoma.
UVC rays are the highest energy, most dangerous type of ultraviolet light. Little attention has been given to UVC rays in the past since they are filtered out by the atmosphere. However, their use in equipment such as pond sterilization units may pose an exposure risk, if the lamp is switched on outside of its enclosed pond sterilization unit.
Skin
| “ | Ultraviolet (UV) irradiation present in sunlight is an environmental human carcinogen. The toxic effects of UV from natural sunlight and therapeutic artificial lamps are a major concern for human health. The major acute effects of UV irradiation on normal human skin comprise sunburn inflammation erythema, tanning, and local or systemic immunosuppression. | ” |
|
— Matsumura and Ananthaswamy , (2004)
|
UVA, UVB and UVC can all damage collagen fibers and thereby accelerate aging of the skin. Both UVA and UVB destroy vitamin A in skin which may cause further damage. In the past UVA was considered less harmful, but today it is known, that it can contribute to skin cancer via the indirect DNA damage (free radicals and reactive oxygen species). It penetrates deeply but it does not cause sunburn. UVA does not damage DNA directly like UVB and UVC, but it can generate highly reactive chemical intermediates, such as hydroxyl and oxygen radicals, which in turn can damage DNA. Because it does not cause reddening of the skin (erythema) it cannot be measured in the SPF testing. There is no good clinical measurement of the blocking of UVA radiation, but it is important that sunscreen block both UVA and UVB. Some scientists blame the absence of UVA filters in sunscreens for the higher melanoma-risk that was found for sunscreen users.
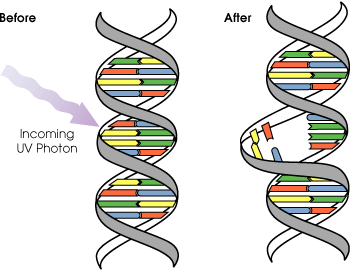
UVB light can cause direct DNA damage. The radiation excites DNA molecules in skin cells, causing aberrant covalent bonds to form between adjacent cytosine bases, producing a dimer. When DNA polymerase comes along to replicate this strand of DNA, it reads the dimer as "AA" and not the original "CC". This causes the DNA replication mechanism to add a "TT" on the growing strand. This is a mutation, which can result in cancerous growths and is known as a "classical C-T mutation". The mutations that are caused by the direct DNA damage carry a UV signature mutation that is commonly seen in skin cancers. The mutagenicity of UV radiation can be easily observed in bacteria cultures. This cancer connection is one reason for concern about ozone depletion and the ozone hole. UVB causes some damage to collagen but at a very much slower rate than UVA.
As a defense against UV radiation, the amount of the brown pigment melanin in the skin increases when exposed to moderate (depending on skin type) levels of radiation; this is commonly known as a sun tan. The purpose of melanin is to absorb UV radiation and dissipate the energy as harmless heat, blocking the UV from damaging skin tissue. UVA gives a quick tan that lasts for days by oxidizing melanin that was already present and triggers the release of the melanin from melanocytes. UVB yields a tan that takes roughly 2 days to develop because it stimulates the body to produce more melanin. The photochemical properties of melanin make it an excellent photoprotectant. However, sunscreen chemicals can not dissipate the energy of the excited state as efficiently as melanin and therefore the penetration of sunscreen ingredients into the lower layers of the skin is increasing the amount of free radicals and ROS.
Sunscreen prevents the direct DNA damage which causes sunburn. Most of these products contain an SPF rating to show how well they block UVB rays. The SPF rating, however, offers no data about UVA protection. In the US, the FDA is considering adding a star rating system to show UVA protection. A similar system is already used in some European countries.
Some sunscreen lotions now include compounds such as titanium dioxide which helps protect against UVA rays. Other UVA blocking compounds found in sunscreen include zinc oxide and avobenzone. Cantaloupe extract, rich in the compound superoxide dismutase (SOD), can be bound with gliadin to form glisodin, an orally-effective protectant against UVB radiation. There are also naturally occurring compounds found in rainforest plants that have been known to protect the skin from UV radiation damage, such as the fern Phlebodium aureum.
Sunscreen safety debate
Medical organizations recommend that patients protect themselves from UV radiation using sunscreen. Five sunscreen ingredients have been shown to protect mice against skin tumors (see sunscreen).
However, some sunscreen chemicals produce potentially harmful substances if they are illuminated while in contact with living cells. The amount of sunscreen which penetrates through the stratum corneum may or may not be large enough to cause damage. In one study of sunscreens, the authors write:
The question whether UV filters acts on or in the skin has so far not been fully answered. Despite the fact that an answer would be a key to improve formulations of sun protection products, many publications carefully avoid addressing this question.
In an experiment that was published in 2006 by Hanson et al, the amount of harmful reactive oxygen species (ROS) had been measured in untreated and in sunscreen treated skin. In the first 20 minutes the film of sunscreen had a protective effect and the number of ROS species was smaller. After 60 minutes however the amount of absorbed sunscreen was so high, that the amount of ROS was higher in the sunscreen treated skin than in the untreated skin.
Eye
High intensities of UVB light are hazardous to the eyes, and exposure can cause welder's flash ( photokeratitis or arc eye) and may lead to cataracts, pterygium, and pinguecula formation.
Protective eyewear is beneficial to those who are working with or those who might be exposed to ultraviolet radiation, particularly short wave UV. Given that light may reach the eye from the sides, full coverage eye protection is usually warranted if there is an increased risk of exposure, as in high altitude mountaineering. Mountaineers are exposed to higher than ordinary levels of UV radiation, both because there is less atmospheric filtering and because of reflection from snow and ice.
Ordinary, untreated eyeglasses give some protection. Most plastic lenses give more protection than glass lenses, because, as noted above, glass is transparent to UVA and the common acrylic plastic used for lenses is less so. Some plastic lens materials, such as polycarbonate, inherently block most UV. There are protective treatments available for eyeglass lenses that need it which will give better protection. But even a treatment that completely blocks UV will not protect the eye from light that arrives around the lens.
Degradation of polymers, pigments and dyes
Many polymers used in consumer products are degraded by UV light, and need addition of UV absorbers to inhibit attack, especially if the products are used externally and so exposed to sunlight. The problem appears as discoloration or fading, cracking and sometimes, total product disintegration if cracking has proceeded far enough. The rate of attack increases with exposure time and sunlight intensity.
It is known as UV degradation, and is one form of polymer degradation. Sensitive polymers include thermoplastics, such as polypropylene and polyethylene as well as speciality fibres like aramids. UV absorption leads to chain degradation and loss of strength at sensitive points in the chain structure. They include tertiary carbon atoms, which in polypropylene occur in every repeat unit.
In addition, many pigments and dyes absorb UV and change colour, so paintings and textiles may need extra protection both from sunlight and fluorescent lamps, two common sources of UV radiation. Old and antique paintings such as watercolour paintings for example, usually need to be placed away from direct sunlight. Common window glass provides some protection by absorbing some of the harmful UV, but valuable artifacts need shielding.
Blockers and absorbers
Ultraviolet Light Absorbers (UVAs) are molecules used in organic materials ( polymers, paints, etc.) to absorb UV light in order to reduce the UV degradation (photo-oxidation) of a material. A number of different UVAs exist with different absorption properties. UVAs can disappear over time, so monitoring of UVA levels in weathered materials is necessary.
In sunscreen, ingredients which absorb UVA/UVB rays, such as avobenzone and octyl methoxycinnamate, are known as absorbers. They are contrasted with physical "blockers" of UV radiation such as titanium dioxide and zinc oxide. (See sunscreen for a more complete list.)
Applications of UV
Security
To help thwart counterfeiters, sensitive documents (e.g. credit cards, driver's licenses, passports) may also include a UV watermark that can only be seen when viewed under a UV-emitting light. Passports issued by most countries usually contain UV sensitive inks and security threads. Visa stamps and stickers on passports of visitors contain large and detailed seals invisible to the naked eye under normal lights, but strongly visible under UV illumination. Passports issued by many nations have UV sensitive watermarks on all pages of the passport. Currencies of various countries' banknotes have an image, as well as many multicolored fibers, that are visible only under ultraviolet light.
Some brands of pepper spray will leave an invisible chemical (UV Dye) that is not easily washed off on a pepper sprayed attacker, which would help police identify them later.
Fluorescent lamps
Fluorescent lamps produce UV radiation by ionising low-pressure mercury vapour. A phosphorescent coating on the inside of the tubes absorbs the UV and converts it to visible light.
The main mercury emission wavelength is in the UVC range. Unshielded exposure of the skin or eyes to mercury arc lamps that do not have a conversion phosphor is quite dangerous.
The light from a mercury lamp is predominantly at discrete wavelengths. Other practical UV sources with more continuous emission spectra include xenon arc lamps (commonly used as sunlight simulators), deuterium arc lamps, mercury-xenon arc lamps, metal-halide arc lamps, and tungsten-halogen incandescent lamps.
Astronomy

In astronomy, very hot objects preferentially emit UV radiation (see Wien's law). Because the ozone layer blocks many UV frequencies from reaching telescopes on the surface of the Earth, most UV observations are made from space. (See UV astronomy, space observatory.)
Biological surveys and pest control
Some animals, including birds, reptiles, and insects such as bees, can see into the near ultraviolet. Many fruits, flowers, and seeds stand out more strongly from the background in ultraviolet wavelengths as compared to human colour vision. Scorpions glow or take on a yellow to green colour under UV illumination. Many birds have patterns in their plumage that are invisible at usual wavelengths but observable in ultraviolet, and the urine and other secretions of some animals, including dogs, cats, and human beings, is much easier to spot with ultraviolet.
Many insects use the ultraviolet wavelength emissions from celestial objects as references for flight navigation. A local ultraviolet emissor will normally disrupt the navigation process and will eventually attract the flying insect.
Ultraviolet traps called bug zappers are used to eliminate various small flying insects. They are attracted to the UV light, and are killed using an electric shock, or trapped once they come into contact with the device. Different designs of ultraviolet light traps are also used by entomologists for collecting nocturnal insects during faunistic survey studies.
Spectrophotometry
UV/VIS spectroscopy is widely used as a technique in chemistry, to analyze chemical structure, most notably conjugated systems. UV radiation is often used in visible spectrophotometry to determine the existence of fluorescence in a given sample.
Analyzing minerals

Ultraviolet lamps are also used in analyzing minerals, gems, and in other detective work including authentication of various collectibles. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light; or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet.
Chemical markers
UV fluorescent dyes are used in many applications (for example, biochemistry and forensics). The Green Fluorescent Protein (GFP) is often used in genetics as a marker. Many substances, such as proteins, have significant light absorption bands in the ultraviolet that are of use and interest in biochemistry and related fields. UV-capable spectrophotometers are common in such laboratories.
Photochemotherapy
Exposure to UVA light while the skin is hyper-photosensitive by taking psoralens is an effective treatment for psoriasis called PUVA. Due to psoralens potentially causing damage to the liver, PUVA may only be used a limited number of times over a patient's lifetime
Phototherapy
Exposure to UVB light, particularly the 310 nm narrowband UVB range, is an effective long-term treatment for many skin conditions like psoriasis, vitiligo, eczema, and others. UVB phototherapy does not require additional medications or topical preparations for the therapeutic benefit; only the light exposure is needed. However, phototherapy can be effective when used in conjunction with certain topical treatments such as anthralin, coal tar, and Vitamin A and D derivatives, or systemic treatments such as methotrexate and soriatane.
Typical treatment regimes involve short exposure to UVB rays 3 to 5 times a week at a hospital or clinic, and for the best results, up to 30 or more sessions may be required.
Side effects may include itching and redness of the skin due to UVB exposure, and possibly sunburn, if patients do not minimize exposure to natural UV rays during treatment days.
Photolithography
Ultraviolet radiation is used for very fine resolution photolithography, a procedure where a chemical known as a photoresist is exposed to UV radiation which has passed through a mask. The light allows chemical reactions to take place in the photoresist, and after development (a step that either removes the exposed or unexposed photoresist), a geometric pattern which is determined by the mask remains on the sample. Further steps may then be taken to "etch" away parts of the sample with no photoresist remaining.
UV radiation is used extensively in the electronics industry because photolithography is used in the manufacture of semiconductors, integrated circuit components and printed circuit boards.
Checking electrical insulation
A new application of UV is to detect corona discharge (often simply called "corona") on electrical apparatus. Degradation of insulation of electrical apparatus or pollution causes corona, wherein a strong electric field ionizes the air and excites nitrogen molecules, causing the emission of ultraviolet radiation. The corona degrades the insulation level of the apparatus. Corona produces ozone and to a lesser extent nitrogen oxide which may subsequently react with water in the air to form nitrous acid and nitric acid vapour in the surrounding air.
Sterilization
Ultraviolet lamps are used to sterilize workspaces and tools used in biology laboratories and medical facilities. Commercially-available low pressure mercury-vapor lamps emit about 86% of their light at 254 nanometers (nm) which coincides very well with one of the two peaks of the germicidal effectiveness curve (i.e., effectiveness for UV absorption by DNA). One of these peaks is at about 265 nm and the other is at about 185 nm. Although 185 nm is better absorbed by DNA, the quartz glass used in commercially-available lamps, as well as environmental media such as water, are more opaque to 185 nm than 254 nm (C. von Sonntag et al., 1992). UV light at these germicidal wavelengths causes adjacent thymine molecules on DNA to dimerize, if enough of these defects accumulate on a microorganism's DNA its replication is inhibited, thereby rendering it harmless (even though the organism may not be killed outright). However, since microorganisms can be shielded from ultraviolet light in small cracks and other shaded areas, these lamps are used only as a supplement to other sterilization techniques.
Disinfecting drinking water
UV radiation can be an effective viricide and bactericide. Disinfection using UV radiation is commonly used in wastewater treatment applications and is finding an increased usage in drinking water treatment. Many bottlers of spring water use UV disinfection equipment to sterilize their water. Solar water disinfection is the process of using PET bottles and sunlight to disinfect water.
New York City has approved the construction of a 2 billion gallon per day ultraviolet drinking water disinfection facility. There are also several facilities under construction and several in operation that treat waste water with several stages of filters, hydrogen peroxide and UV light to bring the water up to drinking standards. One such facility exists in Orange County California.
It used to be thought that UV disinfection was more effective for bacteria and viruses, which have more exposed genetic material, than for larger pathogens which have outer coatings or that form cyst states (e.g., Giardia) that shield their DNA from the UV light. However, it was recently discovered that ultraviolet radiation can be somewhat effective for treating the microorganism Cryptosporidium. The findings resulted in two US patents and the use of UV radiation as a viable method to treat drinking water. Giardia in turn has been shown to be very susceptible to UV-C when the tests were based on infectivity rather than excystation. It has been found that protists are able to survive high UV-C doses but are sterilized at low doses.
Solar water disinfection (SODIS) has been extensively researched in Switzerland and has proven ideal to treat small quantities of water cheaply using natural sunlight. Contaminated water is poured into transparent plastic bottles and exposed to full sunlight for six hours. The sunlight treats the contaminated water through two synergetic mechanisms: UV-A irradiation and increased water temperature. If the water temperatures rises above 50 °C, the disinfection process is three times faster.
Food processing
As consumer demand for fresh and "fresh-like" food products increases, the demand for nonthermal methods of food processing is likewise on the rise. In addition, public awareness regarding the dangers of food poisoning is also raising demand for improved food processing methods. Ultraviolet radiation is used in several food processes to kill unwanted microorganisms. UV light can be used to pasteurize fruit juices by flowing the juice over a high intensity ultraviolet light source. The effectiveness of such a process depends on the UV absorbance of the juice (see Beer's law).
Fire detection
Ultraviolet detectors generally use either a solid-state device, such as one based on silicon carbide or aluminium nitride, or a gas-filled tube as the sensing element. UV detectors which are sensitive to UV light in any part of the spectrum respond to irradiation by sunlight and artificial light. A burning hydrogen flame, for instance, radiates strongly in the 185 to 260 nanometer range and only very weakly in the IR region, while a coal fire emits very weakly in the UV band yet very strongly at IR wavelengths; thus a fire detector which operates using both UV and IR detectors is more reliable than one with a UV detector alone. Virtually all fires emit some radiation in the UVB band, while the Sun's radiation at this band is absorbed by the Earth's atmosphere. The result is that the UV detector is "solar blind", meaning it will not cause an alarm in response to radiation from the Sun, so it can easily be used both indoors and outdoors.
UV detectors are sensitive to most fires, including hydrocarbons, metals, sulfur, hydrogen, hydrazine, and ammonia. Arc welding, electrical arcs, lightning, X-rays used in nondestructive metal testing equipment (though this is highly unlikely), and radioactive materials can produce levels that will activate a UV detection system. The presence of UV-absorbing gases and vapors will attenuate the UV radiation from a fire, adversely affecting the ability of the detector to detect flames. Likewise, the presence of an oil mist in the air or an oil film on the detector window will have the same effect.
Curing of inks, adhesives, varnishes and coatings
Certain inks, coatings and adhesives are formulated with photoinitiators and resins. When exposed to the correct energy and irradiance in the required band of UV light, polymerization occurs, and so the adhesives harden or cure. Usually, this reaction is very quick, a matter of a few seconds. Applications include glass and plastic bonding, optical fibre coatings, the coating of flooring, UV Coating and paper finishes in offset printing, and dental fillings.
An industry has developed around the manufacture of UV lamps sourced for UV curing applictions. Fast processes such as flexo or offset printing require high intensity light focused via reflectors onto a moving substrate and medium and high pressure Hg (mercury) or Fe (iron) based bulbs are used which can be energised with electric arc or microwaves. Lower power fluorescent lamps can be used for static applications and in some cases, small high pressure lamps can have light focused and transmitted to the work area via liquid filled or fibre optic light guides.
Radtech is a trade association dedicated to the promotion of this technology.
Deterring substance abuse in public places
UV lights have been installed in some parts of the world in public restrooms, and on public transport, for the purpose of deterring substance abuse. The blue colour of these lights, combined with the fluorescence of the skin, make it harder for intravenous drug users to find a vein. The efficacy of these lights for that purpose has been questioned, with some suggesting that drug users simply find a vein outside the public restroom and mark the spot with a marker for accessibility when inside the restroom. There is currently no published evidence supporting the idea of a deterrent effect.
Sun tanning
Sun tanning describes a darkening of the skin in a natural physiological response stimulated by exposure to ultraviolet radiation from sunshine (or a sunbed). With excess exposure to the sun, a suntanned area can also develop sunburn. The increased production of melanin is triggered by the direct DNA damage. This kind of damage is recognized by the body and as a defense against UV radiation the skin produces more melanin. Melanin dissipates the UV energy as harmless heat, and therefore it is an excellent photoprotectant. Melanin protects against the direct DNA damage and against the indirect DNA damage. Sunscreen protects only against the direct DNA damage, but increases the indirect DNA damage - this causes the higher amount of melanoma that had been found repeatedly in sunscreen users compared to non-users.
Erasing EPROM modules
Some EPROM (electronically programmable read-only memory) modules are erased by exposure to UV radiation. These modules often have a transparent glass (quartz) window on the top of the chip that allows the UV radiation in. These have been largely superseded by EEPROM and flash memory chips in most devices.
Preparing low surface energy polymers
UV radiation is useful in preparing low surface energy polymers for adhesives. Polymers exposed to UV light will oxidize thus raising the surface energy of the polymer. Once the surface energy of the polymer has been raised, the bond between the adhesive and the polymer will not be smaller.
Reading otherwise illegible papyruses
Using multi-spectral imaging it is possible to read illegible papyruses, such as the burned papyruses of the Villa of the Papyri or of Oxyrhynchus. The technique involves taking pictures of the illegible papyruses using different filters in the infrared or ultraviolet range, finely tuned to capture certain wavelengths of light. Thus, the optimum spectral portion can be found for distinguishing ink from paper on the papyrus surface.
Lasers
Ultraviolet lasers have applications in industry ( laser engraving), medicine ( dermatology and keratectomy), free air secure communications and computing ( optical storage). They can be made by applying frequency conversion to lower-frequency lasers, or from Ce:LiSAF crystals (cerium doped with lithium strontium aluminium fluoride), a process developed in the 1990s at Lawrence Livermore National Laboratory.
Evolutionary significance
Evolution of early reproductive proteins and enzymes is attributed in modern models of evolutionary theory to ultraviolet light. UVB light causes thymine base pairs next to each other in genetic sequences to bond together into thymine dimers, a disruption in the strand which reproductive enzymes cannot copy (see picture above). This leads to frameshifting during genetic replication and protein synthesis, usually killing the organism. As early prokaryotes began to approach the surface of the ancient oceans, before the protective ozone layer had formed, blocking out most wavelengths of UV light, they almost invariably died out. The few that survived had developed enzymes which verified the genetic material and broke up thymine dimer bonds, known as excision repair enzymes. Many enzymes and proteins involved in modern mitosis and meiosis are extremely similar to excision repair enzymes, and are believed to be evolved modifications of the enzymes originally used to overcome UV light.