Vitamin K
2008/9 Schools Wikipedia Selection. Related subjects: Chemical compounds; Food
Vitamin K (K from "Koagulations-Vitamin" in German and Danish) denotes a group of lipophilic, hydrophobic vitamins that are needed for the posttranslational modification of certain proteins, mostly required for blood coagulation. Chemically they are 2- methyl- 1,4-naphthoquinone derivatives.
Vitamin K2 (menaquinone, menatetrenone) is normally produced by bacteria in the intestines, and dietary deficiency is extremely rare unless the intestines are heavily damaged or are unable to absorb the molecule.
Chemical structure
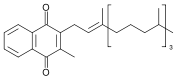
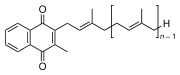
All members of the vitamin K group of vitamins share a methylated naphthoquinone ring structure, and vary in the aliphatic side chain attached at the 3-position (see figure 1). Phylloquinone (also known as vitamin K1) invariably contains in its side chain four isoprenoid residues, one of which is unsaturated.
Menaquinones have side chains composed of a variable number of unsaturated isoprenoid residues; generally they are designated as MK-n, where n specifies the number of isoprenoids.
It is generally accepted that the naphthoquinone is the functional group, so that the mechanism of action is similar for all K-vitamins. Substantial differences may be expected, however, with respect to intestinal absorption, transport, tissue distribution, and bio-availability. These differences are caused by the different lipophilicity of the various side chains, and by the different food matrices in which they occur.
Physiology
Vitamin K is involved in the carboxylation of certain glutamate residues in proteins to form gamma-carboxyglutamate residues (abbreviated Gla-residues). The modified residues are situated within specific protein domains called Gla domains. Gla-residues are usually involved in binding calcium. The Gla-residues are essential for the biological activity of all known Gla-proteins.
At this time 14 human proteins with Gla domains have been discovered, and they play key roles in the regulation of three physiological processes:
- Blood coagulation: ( prothrombin (factor II), factors VII, IX, X, protein C, protein S and protein Z).
- Bone metabolism: osteocalcin, also called bone Gla-protein (BGP), and matrix gla protein (MGP).
- Vascular biology.
Recommended amounts
The U.S. Dietary Reference Intake (DRI) for an Adequate Intake (AI) of Vitamin K for a 25-year old male is 120 micrograms/day. No Tolerable Upper Intake Level (UL) has been set. The human body stores Vitamin K, so it is not necessary to take Vitamin K daily.
Role in disease
Vitamin K-deficiency may occur by disturbed intestinal uptake (such as would occur in a bile duct obstruction), by therapeutic or accidental intake of vitamin K-antagonists or, very rarely, by nutritional vitamin K-deficiency. As a result, Gla-residues are inadequately formed and the Gla-proteins are insufficiently active. Lack of control of the three processes mentioned above may lead to the following: risk of massive, uncontrolled bleeding, cartilage calcification and severe malformation of developing bone, or deposition of insoluble calcium salts in the walls of arteries. The deposition of calcium in soft tissues, including arterial walls, is quite common, especially in those suffering from atherosclerosis, suggesting that Vitamin K deficiency is more common than previously thought. Menaquinone, but not phylloquinone, intake is associated with reduced risk of CHD mortality, all-cause mortality and severe aortic calcification.
Postmenopausal and elderly women in Thailand have high risk of Vitamin K(2) deficiency, comparing to the normal value of young, reproductive females. Current dosage recommendations for Vitamin K may be too low.
Use on newborn babies
In some countries, injections of Vitamin K are routinely given to newborn babies. Vitamin K is used as prophylactic measure to prevent late-onset haemorrhagic disease ( HDN). However, HDN is a relatively rare problem, and many parents now choose for their babies not to have such an injection.
Biochemistry
Discovery
In 1929, Danish scientist Henrik Dam investigated the role of cholesterol by feeding chickens a cholesterol-depleted diet. After several weeks, the animals developed hemorrhages and started bleeding. These defects could not be restored by adding purified cholesterol to the diet. It appeared that - together with the cholesterol - a second compound had been extracted from the food, and this compound was called the coagulation vitamin. The new vitamin received the letter K because the initial discoveries were reported in a German journal, in which it was designated as Koagulationsvitamin. Edward Adelbert Doisy of Saint Louis University did much of the research that led to the discovery of the structure and chemical nature of Vitamin K. Dam and Doisy shared the 1943 Nobel Prize for medicine for their work on Vitamin K. Several laboratories synthesized the compound in 1939.
For several decades the vitamin K-deficient chick model was the only method of quantitating vitamin K in various foods: the chicks were made vitamin K-deficient and subsequently fed with known amounts of vitamin K-containing food. The extent to which blood coagulation was restored by the diet was taken as a measure for its vitamin K content.
The first published report of successful treatment with vitamin K of life-threatening hemorrhage in a jaundiced patient with prothrombin deficiency was made in 1938 at the University of Iowa Department of Pathology by Drs. Harry Pratt Smith, Emory Warner, Kenneth Brinkhous, and Walter Seegers.
Function in the cell
The precise function of vitamin K was not discovered until 1974, when three laboratories (Stenflo et al., Nelsestuen et al., and Magnusson et al.) isolated the vitamin K-dependent coagulation factor prothrombin (Factor II) from cows that received a high dose of a vitamin K antagonist, warfarin. It was shown that while warfarin-treated cows had a form of prothrombin that contained 10 glutamate amino acid residues near the amino terminus of this protein, the normal (untreated) cows contained 10 unusual residues which were chemically identified as gamma-carboxyglutamate, or Gla. The extra carboxyl group in Gla made clear that vitamin K plays a role in a carboxylation reaction during which Glu is converted into Gla.
The biochemistry of how Vitamin K is used to convert Glu to Gla has been elucidated over the past thirty years in academic laboratories throughout the world. Within the cell, Vitamin K undergoes electron reduction to a reduced form of Vitamin K (called Vitamin K hydroquinone) by the enzyme Vitamin K epoxide reductase (or VKOR). Another enzyme then oxidizes Vitamin K hydroquinone to allow carboxylation of Glu to Gla; this enzyme is called the gamma-glutamyl carboxylase or the Vitamin K-dependent carboxylase. The carboxylation reaction will only proceed if the carboxylase enzyme is able to oxidize Vitamin K hydroquinone to vitamin K epoxide at the same time; the carboxylation and epoxidation reactions are said to be coupled reactions. Vitamin K epoxide is then re-converted to Vitamin K by the Vitamin K epoxide reductase. These two enzymes comprise the so-called Vitamin K cycle. One of the reasons why Vitamin K is rarely deficient in a human diet is because Vitamin K is continually recycled in our cells.
Warfarin and other coumadin drugs block the action of the Vitamin K epoxide reductase. This results in decreased concentrations of Vitamin K and Vitamin K hydroquinone in the tissues, such that the carboxylation reaction catalyzed by the glutamyl carboxylase is inefficient. This results in the production of clotting factors with inadequate Gla. Without Gla on the amino termini of these factors, they no longer bind stably to the blood vessel endothelium and cannot activate clotting to allow formation of a clot during tissue injury. As it is impossible to predict what dose of Warfarin will give the desired degree of suppression of the clotting, Warfarin treatment must be carefully monitored to avoid over-dosing. See Warfarin.
Gla-proteins
At present, the following human Gla-containing proteins have been characterized to the level of primary structure: the blood coagulation factors II (prothrombin), VII, IX, and X, the anticoagulant proteins C and S, and the Factor X-targeting protein Z. The bone Gla-protein osteocalcin, the calcification inhibiting matrix gla protein (MGP), the cell growth regulating growth arrest specific gene 6 protein (Gas6), and the four transmembrane Gla proteins (TMGPs) the function of which is at present unknown. Gas6 can function as a growth factor that activates the Axl receptor tyrosine kinase and stimulates cell proliferation or prevents apoptosis in some cells. In all cases in which their function was known, the presence of the Gla-residues in these proteins turned out to be essential for functional activity.
Gla-proteins are known to occur in a wide variety of vertebrates: mammals, birds, reptiles, and fish. The venom of a number of Australian snakes acts by activating the human blood clotting system. Remarkably, in some cases activation is accomplished by snake Gla-containing enzymes that bind to the endothelium of human blood vessels and catalyze the conversion of procoagulant clotting factors into activated ones, leading to unwanted and potentially deadly clotting.
Another interesting class of invertebrate Gla-containing proteins is synthesized by the fish-hunting snail Conus geographus. These snails produce a venom containing hundreds of neuro-active peptides, or conotoxins, which is sufficiently toxic to kill an adult human. Several of the conotoxins contain 2-5 Gla residues.
Function in Bacteria
Many bacteria, such as Escherichia coli found in the large intestine, can synthesize Vitamin K2 (menaquinone), but not Vitamin K1 (phylloquinone). In these bacteria, menaquinone will transfer two electrons between two different small molecules, in a process called anaerobic respiration. For example, a small molecule with an excess of electrons (also called an electron donor) such as lactate, formate, or NADH, with the help of an enzyme, will pass two electrons to a menaquinone. The menaquinone, with the help of another enzyme, will in turn transfer these 2 electrons to a suitable oxidant, such fumarate or nitrate (also called an electron acceptor). Adding two electrons to fumarate or nitrate will convert the molecule to succinate or nitrite + water, respectively. Some of these reactions generate a cellular energy source, ATP, in a manner similar to eukaryotic cell aerobic respiration, except that the final electron acceptor is not molecular oxygen, but say fumarate or nitrate (In aerobic respiration, the final oxidant is molecular oxygen (O2) , which accepts four electrons from an electron donor such as NADH to be converted to water.) Escherichia coli can carry out aerobic respiration and menaquninone-mediated anaerobic respiration.
Carcinogenicity
Vitamin K substances are IARC Group 3 carcinogens. One study conducted in the United Kingdom in 1970 found a nearly two-fold increase of leukaemia in children administered synthetic Vitamin K1 phytomenadione intramuscularly, but later studies have failed to find whether Vitamin K is carcinogenic or not.