Hydrogen peroxide
2008/9 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Hydrogen peroxide | |
|---|---|
| IUPAC name | Dihydrogen dioxide |
| Other names | μ-1κO,2κO’-Dioxidodihydrogen Hydrogen peroxide Hydrogen dioxide Dioxidane |
| Identifiers | |
| CAS number | [7722-84-1] |
| RTECS number | MX0900000 |
| Properties | |
| Molecular formula | H2O2 |
| Molar mass | 34.0147 g·mol·−1. |
| Appearance | Very pale blue colour; colorless in solution |
| Density | 1.4 g/cm3, liquid |
| Melting point |
-11 °C (262.15 K) |
| Boiling point |
150.2 °C (423.35 K) |
| Solubility in water | Miscible |
| Acidity (pKa) | 11.65 |
| Viscosity | 1.245 c P at 20 °C |
| Dipole moment | 2.26 D |
| Hazards | |
| MSDS | 30% hydrogen peroxide msds 60% hydrogen peroxide msds |
| Main hazards | Oxidant, corrosive |
| NFPA 704 | |
| R-phrases | R5, R8, R20, R22, R35 |
| S-phrases | (S1), (S2), S17, S26, S28, S36, S37, S39, S45 |
| Flash point | Non-flammable |
| Related compounds | |
| Related compounds | Water Ozone Hydrazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references |
|
Hydrogen peroxide (H2O2) is a very pale blue liquid which appears colorless in a dilute solution, slightly more viscous than water. It is a weak acid. It has strong oxidizing properties and is therefore a powerful bleaching agent that is mostly used for bleaching paper, but has also found use as a disinfectant, as an oxidizer, as an antiseptic, and in rocketry (particularly in high concentrations as high-test peroxide (HTP)) as a monopropellant, and in bipropellant systems. The oxidizing capacity of hydrogen peroxide is so strong that the chemical is considered a highly reactive oxygen species.
History
Hydrogen peroxide was first isolated in 1818 by Louis Jacques Thénard by reacting barium peroxide with nitric acid. An improved version of this process used hydrochloric acid, followed by sulfuric acid to precipitate the barium sulfate byproduct. Thénard's process was used from the end of the 19th century until the middle of the 20th century. Modern production methods are discussed below.
Uses
Industrial applications
About 50% of the world's production of hydrogen peroxide in 1994 was used for pulp- and paper-bleaching. Other bleaching applications are becoming more important as hydrogen peroxide is seen as an environmentally benign alternative to chlorine-based bleaches. It is highly corrosive to metal.
Other major industrial applications for hydrogen peroxide include the manufacture of sodium percarbonate and sodium perborate, used as mild bleaches in laundry detergents. It is used in the production of certain organic peroxides such as dibenzoyl peroxide, used in polymerisations and other chemical processes. Hydrogen peroxide is also used in the production of epoxides such as propylene oxide. Reaction with carboxylic acids produces a corresponding peroxy acid. Peracetic acid and meta-chloroperoxybenzoic acid (commonly abbreviated mCPBA) are prepared from acetic acid and meta-chlorobenzoic acid, respectively. The latter is commonly reacted with alkenes to give the corresponding epoxide.
In the PCB manufacturing process, hydrogen peroxide mixed with sulfuric acid was used as the microetch chemical for copper surface roughening preparation.
A combination of a powdered precious metal-based catalyst, hydrogen peroxide, methanol and water can produce superheated steam in one to two seconds, releasing only CO2 and high temperature steam for a variety of purposes..
Domestic uses
- Diluted H2O2 (around 15%) is used to bleach human hair, hence the phrase "peroxide blonde". It is absorbed by skin upon contact and creates a local skin capillary embolism which appears as a temporary whitening of the skin. It is used to whiten bones that are to be put on display. The strength of a solution may be described as a percentage or volume, where 1% hydrogen peroxide releases 3.3 volumes of oxygen during decomposition.Thus, a 3% solution is equivalent to 10 volume and a 6% solution to 20 volume, etc.
- 3% H2O2 is used medically for cleaning wounds, removing dead tissue, and as an oral debriding agent. Peroxide stops slow (small vessel) wound bleeding/oozing, as well. Most over-the-counter peroxide solutions are not suitable for ingestion.
- 3% H2O2 is effective at treating fresh (red) blood-stains in clothing and on other items. It must be applied to clothing before blood stains can be accidentally "set" with heated water. Cold water and soap are then used to remove the peroxide treated blood.
- The Food and Drug Administration (FDA) has classified hydrogen peroxide as a Low Regulatory Priority (LRP) drug for use in controlling fungus on fish and fish eggs. (See ectoparasite.)
- Some gardeners and users of hydroponics advocate the use of hydrogen peroxide in watering solutions. They claim that its spontaneous decomposition releases oxygen that enhances a plant's root development and helps to treat root rot (cellular root death due to lack of oxygen).
- Laboratory tests conducted by fish culturists in recent years have demonstrated that common household hydrogen peroxide can be used safely to provide oxygen for small fish. Hydrogen peroxide releases oxygen by decomposition when it is exposed to catalysts such as manganese dioxide.
- Hydrogen peroxide is a strong oxidizer effective in controlling sulfide and organic related odors in wastewater collection and treatment systems. It is typically applied to a wastewater system where there is a retention time of 30 minutes to 5 hours before hydrogen sulfide is released. Hydrogen peroxide oxidizes the hydrogen sulfide and promotes bio-oxidation of organic odours. Hydrogen peroxide decomposes to oxygen and water, adding dissolved oxygen to the system thereby negating some Biological Oxygen Demand (BOD).
- Hydrogen peroxide is used with phenyl oxalate ester and an appropriate dye in glow sticks as an oxidizing agent. It reacts with the ester to form an unstable CO2 dimer which excites the dye to an excited state; the dye emits a photon (light) when it spontaneously relaxes back to the ground state.
Storage
Regulations vary, but low concentrations, such as 2.5% are widely available and legal to buy for medical use. Small quantities of many different concentrations and grades can be legally stored and used with few regulations.
Hydrogen peroxide should be stored in a container made from a material that it doesn't react with and doesn't catalyze its decomposition. Numerous materials and processes are available, some stainless steels, many plastics, glasses and some aluminium alloys are compatible.
Peroxide is a strong oxidant and should be stored away from fuel sources and sources of catalytic contamination (see decomposition section). Apart from obvious fire risks, peroxide vapour can react with hydrocarbons and alcohols to form contact explosives. Because oxygen is formed during the natural decomposition of the peroxide, the resulting increase in pressure can cause a container (e.g. made of glass) to shatter. Peroxide should be kept cool, as peroxide vapour can detonate above 70 °C. Deaths have occurred from storage in inadequately labeled containers due to its apparent similarity to water.
Use as propellant
H2O2 can be used either as a monopropellant (not mixed with fuel) or as the oxidizer component of a bipropellant rocket. Use as a monopropellant takes advantage of the decomposition of 70–98+% concentration hydrogen peroxide into steam and oxygen. The propellant is pumped into a reaction chamber where a catalyst, usually a silver or platinum screen, triggers decomposition, producing steam at over 600 °C which is expelled through a nozzle, generating thrust. H2O2 monopropellant produces a maximum specific impulse (Isp) of 161 s (1.6 kN·s/kg), which makes it a low-performance monopropellant. Peroxide generates much less thrust than toxic hydrazine, but is not toxic. The Bell Rocket Belt used hydrogen peroxide monopropellant.
As a bipropellant H2O2 is decomposed to burn a fuel as an oxidizer. Specific impulses as high as 350 s (3.5 kN·s/kg) can be achieved, depending on the fuel. Peroxide used as an oxidizer gives a somewhat lower Isp than liquid oxygen, but is dense, storable, noncryogenic and can be more easily used to drive gas turbines to give high pressures. It can also be used for regenerative cooling of rocket engines. Peroxide was used very successfully as an oxidizer in World-War-II German rockets (e.g. T-Stoff for the Me-163), and for the low-cost British Black Knight and Black Arrow launchers.
In the 1940s and 1950s the Walter turbine used hydrogen peroxide for use in submarines while submerged; it was found to be too noisy and require too much maintenance compared to diesel-electric power systems. Some torpedoes used hydrogen peroxide as oxidizer or propellant, but this was dangerous and has been discontinued by most navies. Hydrogen peroxide leaks were blamed for the sinkings of HMS Sidon and the Russian submarine Kursk. It was discovered, for example, by the Japanese Navy in torpedo trials, that the concentration of H2O2 in right-angle bends in HTP pipework can often lead to explosions in submarines and torpedoes. Hydrogen peroxide is still used on Soyuz for driving gas turbines to power turbopumps, however. SAAB Underwater Systems is manufacturing the Torpedo 2000. This torpedo, used by the Swedish navy, is powered by a piston engine propelled by HTP as an oxidizer and kerosene as a fuel in a bipropellant system.
While rarely used now as a monopropellant for large engines, small hydrogen peroxide attitude control thrusters are still in use on some satellites. They are easy to throttle, and safer to fuel and handle before launch than hydrazine thrusters. However, hydrazine is more often used in spacecraft because of its higher specific impulse and lower rate of decomposition.
Recently H2O2/ propylene has been proposed as an approach to inexpensive Single Stage To Orbit: a fuel tank containing propylene has a bladder floating in it containing H2O2. This combination offers 15% superior Isp to O2/RP4 (a kerosene used as rocket propellant), does not need turbines or cryogenic storage or hardware, and greatly reduces the cost of the booster. The potential of this and other alternative systems is discussed in some detail at Dunn Engineering.
Therapeutic use
Hydrogen peroxide is generally recognized as safe (GRAS) as an antimicrobial agent, an oxidizing agent and for other purposes by the US Food and Drug Administration.
Hydrogen peroxide has been used as an antiseptic and anti-bacterial agent for many years due to its oxidizing effect. While its use has decreased in recent years with the popularity of better-smelling and more readily-available over the counter products, it is still used by many hospitals, doctors and dentists in sterilizing, cleaning and treating everything from floors to root canal procedures.
- Like many oxidative antiseptics, hydrogen perioxide causes mild damage to tissue in open wounds, but it also is effective at rapidly stopping capillary bleeding (slow blood oozing from small vessels in abrasions), and is sometimes used sparingly for this purpose, as well as cleaning.
- Hydrogen peroxide can be used as a toothpaste when mixed with correct quantities of baking soda and salt.
- Hydrogen peroxide and benzoyl peroxide are sometimes used to treat acne.
- Hydrogen peroxide is used as an emetic in veterinary practice.
- "Alternative" uses
- Some people have tried using peroxide as a treatment for cancer. The American Cancer Society states that "there is no scientific evidence that hydrogen peroxide is a safe, effective or useful cancer treatment", and advises cancer patients to "remain in the care of qualified doctors who use proven methods of treatment and approved clinical trials of promising new treatments."
- Another controversial alternative medical procedure is inhalation of hydrogen peroxide at a concentration of about 1%. Internal use of hydrogen peroxide has a history of causing fatal blood disorders, and its recent use as a therapeutic treatment has been linked to several deaths.
Physical properties
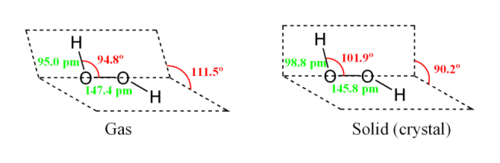
While the anti conformer would minimize steric repulsions, a 90° torsion angle would optimize mixing between the filled p-type orbital of the oxygen (one of the lone pairs) and the LUMO of the vicinal O-H bond. Reflecting a compromise between the two interactions, gaseous and liquid hydrogen peroxide adopts an anticlinal "skewed" shape. This rotational conformation is a compromise between the anti conformer, which would minimize steric repulsion, and between the syn conformer that associates O-H bonds with lone pairs on the oxygen atoms. Despite the fact that the O-O bond is a single bond, the molecule has a remarkably high barrier to complete rotation of 29.45 kJ/ mol (compared with 12.5 kJ/mol for the rotational barrier of ethane). The increased barrier is also attributed to repulsion between one lone pair and other lone pairs. The bond angles are affected by hydrogen bonding, which is relevant to the structural difference between gaseous and crystalline forms; indeed a wide range of values is seen in crystals containing molecular H2O2.
Chemical properties
H2O2 is one of the most powerful oxidizers known -- stronger than chlorine, chlorine dioxide, and potassium permanganate. Also, through catalysis, H2O2 can be converted into hydroxyl radicals (.OH) with reactivity second only to fluorine.
| Oxidant | Oxidation potential, V |
|---|---|
| Fluorine | 3.0 |
| Hydroxyl radical | 2.8 |
| Ozone | 2.1 |
| Hydrogen peroxide | 1.8 |
| Potassium permanganate | 1.7 |
| Chlorine dioxide | 1.5 |
| Chlorine | 1.4 |
Hydrogen peroxide can decompose spontaneously into water and oxygen. It usually acts as an oxidizing agent, but there are many reactions where it acts as a reducing agent, releasing oxygen as a by-product.
It also readily forms both inorganic and organic peroxides.
Decomposition
Hydrogen peroxide always decomposes (disproportionates) exothermically into water and oxygen gas spontaneously:
- 2 H2O2 → 2 H2O + O2
This process is very favorable; it has a ΔHo of −98.2 kJ· mol−1 and a ΔGo of −119.2 kJ·mol−1 and a ΔS of 70.5 J·mol−1·K−1. The rate of decomposition is dependent on the temperature and concentration of the peroxide, as well as the pH and the presence of impurities and stabilizers. Hydrogen peroxide is incompatible with many substances that catalyse its decomposition, including most of the transition metals and their compounds. Common catalysts include manganese dioxide, and silver. The same reaction is catalysed by the enzyme catalase, found in the liver, whose main function in the body is the removal of toxic byproducts of metabolism and the reduction of oxidative stress. The decomposition occurs more rapidly in alkali, so acid is often added as a stabilizer.
The liberation of oxygen and energy in the decomposition has dangerous side effects. Spilling high concentration peroxide on a flammable substance can cause an immediate fire, which is further fueled by the oxygen released by the decomposing hydrogen peroxide. High-strength peroxide (also called high-test peroxide, or HTP) must be stored in a suitable, vented container to prevent the buildup of oxygen gas, which would otherwise lead to the eventual rupture of the container.
In the presence of certain catalysts, such as Fe2+ or Ti3+, the decomposition may take a different path, with free radicals such as HO· ( hydroxyl) and HOO· being formed. A combination of H2O2 and Fe2+ is known as Fenton's reagent.
A common concentration for hydrogen peroxide is "20 volume", which means that when 1 volume of hydrogen peroxide is decomposed, it produces 20 volumes of oxygen. A 20 "volume" concentration of hydrogen peroxide is equivalent to 1.67 mol/dm3 ( Molar solution) or about 6%.
Hydrogen peroxide available at drug stores is three percent solution. In such small concentrations, it is less stable, and decomposes faster. It is usually stabilized with acetanilide, a substance that has toxic side effects in significant amounts.
Redox reactions
In aqueous solution, hydrogen peroxide can oxidize or reduce a variety of inorganic ions. When it acts as a reducing agent, oxygen gas is also produced. In acid solution Fe2+ is oxidized to Fe3+,
and sulfite (SO32−) is oxidized to sulfate (SO42−). However, potassium permanganate is reduced to Mn2+ by acidic H2O2. Under alkaline conditions, however, some of these reactions reverse; for example, Mn2+ is oxidized to Mn4+ (as MnO2).
Another example of hydrogen peroxide acting as a reducing agent is the reaction with sodium hypochlorite, this is a convenient method for preparing oxygen in the laboratory.
- NaOCl + H2O2 → O2 + NaCl + H2O
Hydrogen peroxide is frequently used as an oxidizing agent in organic chemistry. One application is for the oxidation of thioethers to sulfoxides. For example, methyl phenyl sulfide was oxidised to methyl phenyl sulfoxide in 99% yield in methanol in 18 hours (or 20 minutes using a TiCl3 catalyst):
- Ph-S-CH3 + H2O2 → Ph-S(O)-CH3 + H2O
Alkaline hydrogen peroxide is used for epoxidation of electron-deficient alkenes such as acrylic acids, and also for oxidation of alkylboranes to alcohols, the second step of hydroboration-oxidation.
Formation of peroxide compounds
Hydrogen peroxide is a weak acid, and it can form hydroperoxide or peroxide salts or derivatives of many metals.
For example, on addition to an aqueous solution of chromic acid (CrO3) or acidic solutions of dichromate salts, it will form an unstable blue peroxide CrO(O2)2. In aqueous solution it rapidly decomposes to form oxygen gas and chromium salts.
It can also produce peroxoanions by reaction with anions; for example, reaction with borax leads to sodium perborate, a bleach used in laundry detergents:
- Na2B4O7 + 4 H2O2 + 2 NaOH → 2 Na2B2O4(OH)4 + H2O
H2O2 converts carboxylic acids (RCOOH) into peroxy acids (RCOOOH), which are themselves used as oxidizing agents. Hydrogen peroxide reacts with acetone to form acetone peroxide, and it interacts with ozone to form hydrogen trioxide. Reaction with urea produces carbamide peroxide, used for whitening teeth. An acid-base adduct with triphenylphosphine oxide is a useful "carrier" for H2O2 in some reactions.
Hydrogen peroxide reacts with ozone to form trioxidane.
Alkalinity
Hydrogen peroxide is a much weaker base than water, but it can still form adducts with very strong acids. The superacid HF/SbF5 forms unstable compounds containing the [H3O2]+ ion.
Manufacture
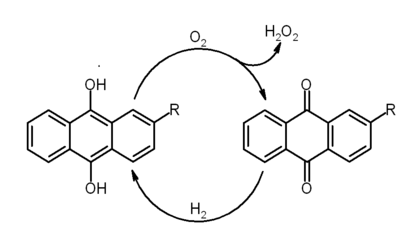
Hydrogen peroxide is manufactured today almost exclusively by the autoxidation of 2-ethyl-9,10-dihydroxyanthracene (C16H14O2) to 2-ethylanthraquinone (C16H12O2) and hydrogen peroxide using oxygen from the air.
In this reaction, the hydroxy groups on the middle ring of anthracene are deprotonated and are turned into ketones, while two double bonds are lost from the middle ring and are replaced as C=O double bonds in the ketone groups. The anthraquinone derivative is then extracted out and reduced back to the dihydroxy compound using hydrogen gas in the presence of a metal catalyst. The overall equation for the process is deceptively simple:
- H2 + O2 → H2O2
However the economics of the process depend on effective recycling of the quinone and extraction solvents, and of the hydrogenation catalyst.
Formerly inorganic processes were used, employing the electrolysis of an aqueous solution of sulfuric acid or acidic ammonium bisulfate (NH4HSO4), followed by hydrolysis of the peroxydisulfate ((SO4)2)2− which is formed.
In 1994, world production of H2O2 was around 1.9 million tonnes and grew to 2.2 million in 2006, most of which was at a concentration of 70% or less. In that year bulk 30% H2O2 sold for around US $0.54 per kg, equivalent to US $1.50 per kg (US $0.68 per lb) on a "100% basis".
Concentration
Hydrogen peroxide works best as a propellant in extremely high concentrations-- roughly over 70%. Although any concentration of peroxide will generate some hot gas (oxygen plus some steam), at concentrations above approximately 67%, the heat of decomposing hydrogen peroxide becomes large enough to completely vaporize all the liquid at standard temperature. This represents a safety and utilization turning point, since decomposition of any concentration above this amount is capable of transforming the liquid entirely to heated gas (the higher the concentration, the hotter the resulting gas). This very hot steam/oxygen mixture can then be used to generate maximal thrust, power, or work, but it also makes explosive decomposition of the material far more hazardous.
Normal propellant grade concentrations therefore vary from 70 to 98%, with common grades of 70, 85, 90, and 98%. Many of these grades and variations are described in detail in the United States propellant specification number MIL-P-16005 Revision F, which is currently available. The available suppliers of high concentration propellant grade hydrogen peroxide are generally one of the large commercial companies which make other grades of hydrogen peroxide; including Solvay Interox, FMC, Degussa and Peroxide Propulsion. Other companies which have made propellant grade hydrogen peroxide in the recent past include Air Liquide and DuPont. DuPont recently sold its hydrogen peroxide manufacturing business to Degussa.
Propellant grade hydrogen peroxide is available to qualified buyers. Typically this chemical is only sold to commercial companies or government institutions which have the ability to properly handle and utilize the material. Non-professionals have purchased 70% or lower concentration hydrogen peroxide (the remaining 30% is water with traces of impurities and stabilizing materials, such as tin salts, phosphates, nitrates, and other chemical additives), and increased its concentration themselves. Many amateurs try distillation, but this is extremely dangerous with hydrogen peroxide; peroxide vapor can ignite or detonate depending on specific combinations of temperature and pressure. In general any boiling mass of high concentration hydrogen peroxide at ambient pressure will produce vapor phase hydrogen peroxide which can detonate. This hazard is mitigated, but not entirely eliminated with vacuum distillation. Other approaches for concentrating hydrogen peroxide are sparging and fractional crystallization.
High concentration hydrogen peroxide is readily available in 70, 90, and 98% concentrations in sizes of 1 gallon, 30 gallon, and bulk tanker truck volumes. Propellant grade hydrogen peroxide is being used on current military systems and is in numerous defense and aerospace research and development programs. Many privately funded rocket companies are using hydrogen peroxide, notably Blue Origin, and some amateur groups have expressed interest in manufacturing their own peroxide, for their use and for sale in small quantities to others.
Hazards
Hydrogen peroxide, either in pure or diluted form, can pose several risks:
- Above roughly 10% concentrations, hydrogen peroxide can give off vapor that can detonate above 50 °C (158 °F) at normal atmospheric pressure. This can then cause a boiling liquid expanding vapor explosion ( BLEVE) of the remaining liquid. Distillation of hydrogen peroxide at normal pressures is thus highly dangerous.
- Hydrogen peroxide vapors can form sensitive contact explosives with hydrocarbons such as greases. Hazardous reactions ranging from ignition to explosion have been reported with alcohols, ketones, carboxylic acids (particularly acetic acid), amines and phosphorus. The saying is 'peroxides kill chemists'.
- Hydrogen peroxide, if spilled on clothing (or other flammable materials), will preferentially evaporate water until the concentration reaches sufficient strength, then clothing will spontaneously ignite. ;
- Concentrated hydrogen peroxide (>50%) is corrosive, and even domestic-strength solutions can cause irritation to the eyes, mucous membranes and skin. Swallowing hydrogen peroxide solutions is particularly dangerous, as decomposition in the stomach releases large quantities of gas (10 times the volume of a 3% solution) leading to internal bleeding. Inhaling over 10% can cause severe pulmonary irritation.
- Low concentrations of hydrogen peroxide, on the order of 3% or less, will chemically bleach many types of clothing it comes into contact with to a pinkish hue. Caution should be exercised when using common products that may contain hydrogen peroxide, such as facial cleaner or contact lens solution, which easily splatter upon other surfaces.
Hydrogen peroxide is naturally produced as a byproduct of oxygen metabolism, and virtually all organisms possess enzymes known as peroxidases, which apparently harmlessly catalytically decomposes low concentrations of hydrogen peroxide to water and oxygen (see Decomposition above).
In one incident, several people were injured after a hydrogen peroxide spill on board Northwest Airlines Flight 957 because they mistook it for water.
During the Second World War some extermination camps experimentally killed people with hydrogen peroxide injections.
Hydrogen peroxide was also part of the ingredients in the July 21, 2005 London Underground bombs, which failed to explode.
An MSDS will contain more information on the risks of working with this chemical.