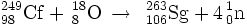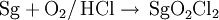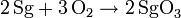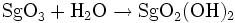Seaborgium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | seaborgium, Sg, 106 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 6, 7, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | unknown, probably silvery white or metallic gray |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [271] g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 7s2 5f14 6d4 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 12, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | presumably a solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | unknown g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | unknown | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | unknown pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | unknown pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 54038-81-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Seaborgium (pronounced /siːˈbɔrgiəm/) is a chemical element in the periodic table that has the symbol Sg and atomic number 106, Image of Seaborgium [ ].
Seaborgium is a synthetic element whose most stable isotope 271Sg has a half-life of 1.9 minutes. Chemistry experiments with seaborgium have firmly placed it in group 6 as a heavier homologue to tungsten.
Official discovery
Element 106 was officially discovered in June 1974 by an American research team led by Albert Ghiorso at the Lawrence Radiation Laboratory at the University of California, Berkeley. They reported creating 263106, with a half-life of 1.0 s, by the hot fusion reaction:
A team led by Ken Gregorich at LBNL confirmed the synthesis in 1994.
The IUPAC/IUPAP Transfermium Working Group (TWG) officially recognised the LBNL team as discoverers in their 1992 report.
Proposed names
Historically, element 106 has been referred to as eka-tungsten.
The Berkeley team suggested the name seaborgium (Sg) to honour the American chemist Glenn T. Seaborg credited as a member of the American group in recognition of his participation in the discovery of several other actinides. The name selected by the team became controversial. An element naming controversy erupted and as a result IUPAC adopted unnilhexium (pronounced /ˌjuːnɪlˈhɛksiəm/ or /ˌʌnɪlˈhɛksiəm/, symbol Unh) as a temporary, systematic element name. In 1994 a committee of IUPAC recommended that element 106 be named rutherfordium and adopted a rule that no element can be named after a living person. This ruling was fiercely objected to by the American Chemical Society. Critics pointed out that a precedent had been set in the naming of einsteinium during Albert Einstein's life and a survey indicated that chemists were not concerned with the fact that Seaborg was still alive. In 1997, as part of a compromise involving elements 104 to 108, the name seaborgium for element 106 was recognized internationally.
Electronic structure
Seaborgium is element 106 in the Periodic Table. The two forms of the projected electronic structure are:
Bohr model: 2, 8, 18, 32, 32, 12, 2
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d4
Extrapolated chemical properties of eka-tungsten/dvi-molybdenum
Oxidation states
Element 106 is projected to be the third member of the 6d series of transition metals and the heaviest member of group VI in the Periodic Table, below chromium,molybdenum and tungsten. All the members of the group readily portray their group oxidation state of +VI and the state becomes more stable as the group is descended. Thus seaborgium is expected to form a stable +VI state. For this group, stable +V and +IV states are well represented for the heavier members and the +III state is known but reducing, unlike Cr(III).
Chemistry
Molybdenum and tungsten readily form stable trioxides MO3 Therefore, seaborgium should from SgO3. The oxides MO3 are soluble in alkali with the formation of oxyanions, so seaborgium should form a seaborgate ion, SgO42-. In addition, WO3 reacts with acid so again eka-tungsten reactivity will show a lack of amphotericity for SgO3. Molybdenum oxide, MoO3, also reacts with moisture to form a hydroxide MoO2(OH)2, so SgO2(OH)2 is also feasible. The heavier homologues readily form the volatile, reactive hexahalides MX6 (X=Cl,F). Only tungsten forms the unstable hexabromide, WBr6. Therefore, the compounds SgF6 and SgCl6 are predicted. An eka-tungsten character may show itself in the formation of a hexabromide, SgBr6. These halides are unstable to oxygen and moisture and readily form volatile oxyhalides, MOX4 and MO2X2. Therefore SgOX4 (X=F,Cl) and SgO2X2 (X=F,Cl) should be possible. In aqueous solution, a variety of anionic oxyfluoro-complexes are formed with fluoride ion, examples being MOF5- and MO3F33-. Similar seaborgium complexes are expected.
Experimental chemistry
Gas phase chemistry
Initial experiments aiming at probing the chemistry of seaborgium focused on the study of the gas thermochromatography of a volatile oxychloride. Seaborgium atoms were produced in the reaction 248Cm(22Ne,4n)266Sg, thermalised and reacted with an O2/HCl mixture. The resulting adsorption properties of the oxychloride was measured and compared with those of molybdenum and tungsten. The result indicated that seaborgium formed a volatile oxychloride in a manner akin to the group 6 elements:
In 2001, a team continued the study of the gas phase chemsitry of seaborgium by reacting the element with O2 in a H2O environment. In a manner similar to the formation of the oxychloride, the results of the experiment indicated the formation of seaborgium oxide hydroxide, a reaction well-known with lighter group 6 homologues.
Aqueous phase chemistry
In its aqueous chemistry, seaborgium has been shown to resemble its lighter homologues molybdenum and tungsten in group 6, forming a stable +6 oxidation state. Seaborgium was eluted from cation exchange resin using a HNO3/HF solution, most likely as neutral SgO2F2 or the anionic complex ion [SgO2F3]-. In contrast, in 0.1 M HNO3, seaborgium does not elute, unlike Mo and W, indicating that the hydrolysis of [Sg(H2O)6]6+ only proceeds as far as the cationic complex [Sg(OH)5(H2O]+.
Summary of investigated compounds and complex ions
| Formula | Names(s) |
|---|---|
| SgO2Cl2 | seaborgium oxychloride ; seaborgium(VI) dioxide dichloride ; seaborgyl dichloride |
| SgO2F2 | seaborgium oxyfluoride ; seaborgium(VI) dioxide difluoride ; seaborgyl difluoride |
| SgO3 | seaborgium oxide ; seaborgium(VI) oxide ; seaborgium trioxide |
| SgO2(OH)2 | seaborgium oxide hydroxide ; seaborgium(VI) dioxide dihydroxide |
| [SgO2F3]- | trifluorodioxoseaborgate(VI) |
| [Sg(OH)5(H2O)]+ | aquapentahydroxyseaborgium(VI) |
History of synthesis of isotopes by cold fusion
208Pb(54Cr,xn)262-xSg (x=1,2,3)
The first attempt to synthesise element 106 in cold fusion reactions was performed in September 1974 by a Soviet team led by G. N. Flerov at the Joint Institute for Nuclear Research at Dubna. They reported producing a 0.48 s spontaneous fission (SF) activity which they assigned to the isotope 259106. Based on later evidence it was suggested that the team most likely measured the decay of 260Sg and its daughter 256Rf. The TWG concluded that, at the time, the results were insufficiently convincing.
The Dubna team revisited this problem in 1983-1984 and were able to detect a 5 ms SF activity assigned directly to 260Sg.
The team at GSI studied this reaction for the first time in 1985 using the improved method of correlation of genetic parent-daughter decays. They were able to detect 261Sg (x=1) and 260Sg and measured a partial 1n neutron evaporation excitation function.
In December 2000, the reaction was studied by a team at GANIL, France and were able to detect 10 atoms of 261Sg and 2 atoms of 260Sg to add to previous data on the reaction.
After a facility upgrade, the GSI team measured the 1n excitation function in 2003 using a metallic lead target. Of significance, in May 2003, the team successfully replaced the lead-208 target with more resistant lead(II) sulfide targets (PbS) which will allow more intense beams to be used in the future. They were able to measure the 1n,2n and 3n excitation functions and performed the first detailed alpha-gamma spectroscopy on the isotope 261Sg. They detected ~1600 atoms of the isotope and identified new alpha lines as well as measuring a more accurate half-life and new EC and SF branchings. Furthermore, they were able to detect the K X-rays from the daughter rutherfordium element for the first time. They were also able to provide improved data for 260Sg, including the tentative observation of an isomeric level. The study was continued in September 2005 and March 2006. The accumulated work on 261Sg was published in 2007. Work in September 2005 also aimed to begin spectroscopic studies on 260Sg.
207Pb(54Cr,xn)261-xSg (x=1,2)
The team at Dubna also studied this reaction in 1974 with identical results as for their first experiments with a Pb-208 target. The SF activities were first assigned to 259Sg and later to 260Sg and/or 256Rf. Further work in 1983-1984 also detected a 5 ms SF activity assigned to the parent 260Sg.
The GSI team studied this reaction for the first time in 1985 using the method of correlation of genetic parent-daughter decays. They were able to positively identify 259Sg as a product from the 2n neutron evaporation channel.
The reaction was further used in March 2005 using PbS targets to begin a spectroscopic study of the even-even isotope 260Sg.
206Pb(54Cr,xn)260-xSg
This reaction was studied in 1974 by the team at Dubna. It was used to assist them in their assignment of the observed SF activities in reactions using Pb-207 and Pb-208 targets. They were unable to detect any SF, indicating the formation of isotopes decaying primarily by alpha decay.
208Pb(52Cr,xn)260-xSg (x=1,2)
The team at Dubna also studied this reaction in their series of cold fusion reactions performed in 1974. Once again they were unable to detect any SF activities. The reaction was revisited in 2006 by the team at LBNL as part of their studies on the effect of the isospin of the projectile and hence the mass number of the compound nucleus on the yield of evaporation residues. They were able to identify 259Sg and 258Sg in their measurement of the 1n excitation function.
209Bi(51V,xn)260-xSg (x=2)
The team at Dubna also studied this reaction in their series of cold fusion reactions performed in 1974. Once again they were unable to detect any SF activities. In 1994, the synthesis of seaborgium was revisited using this reaction by the GSI team, in order to study the new even-even isotope 258Sg. Ten atoms of 258Sg were detected and decayed by spontaneous fission.
History of synthesis of isotopes by hot fusion
238U(30Si,xn)268-xSg (x=3,4,5,6)
This reaction was first studied by Japanese scientists at the Japan Atomic Energy Research Institute (JAERI) in 1998. They detected a spontaneous fission activity which they tentatively assigned to the new isotope 264Sg or 263Db, formed by EC of 263Sg. In 2006, the teams at GSI and LBNL both studied this reaction using the method of correlation of genetic parent-daughter decays. The LBNL team measured an excitation function for the 4n,5n and 6n channels, whilst the GSI team were able to observe an additional 3n activity. Both teams were able to identify the new isotope 264Sg which decayed with a short lifetime by spontaneous fission.
248Cm(22Ne,xn)270-xSg (x=4,5)
In 1993, at Dubna, Yuri Lazarev and his team announced the discovery of long-lived 266Sg and 265Sg produced in the 4n and 5n channels of this nuclear reaction following the search for seaborgium isotopes suitable for a first chemical study. It was announced that 266Sg decayed by 8.57 MeV alpha-particle emission with a projected half-life of ~20 s, lending strong support to the stabilising effect of the Z=108,N=162 closed shells. This reaction was studied further in 1997 by a team at GSI and the yield, decay mode and half-lives for 266Sg and 265Sg have been confirmed, although there are still some discrepancies. In the recent synthesis of 270Hs (see hassium), 266Sg was found to undergo exclusively SF with a short half-life (TSF = 360 ms). It is probable that this is the ground state, (266gSg) and that the other activity, produced directly, belongs to a high spin K-isomer, 266mSg, but further results are required to confirm this.
However, this reaction has been successfully used in the recent attempts to study the chemistry of seaborgium (see below).
249Cf(18O,xn)267-xSg (x=4)
The synthesis of element 106 was first attempted in 1974 by the team at LBNL. In their discovery experiment, they were able to apply the new method of correlation of genetic parent-daughter decays to identify the new isotope 263Sg. In 1975, the team at Oak Ridge were able to confirm the decay data but were unable to identify coincident X-rays in order to prove that seaborgium as produced. In 1979, the team at Dubna studied the reaction by detection of SF activities. In comparison with data from Berkeley, they calculated a 70% SF branching for 263Sg. The synthesis and discovery reaction was confirmed in 1994 by a different team at LBNL.
Synthesis of isotopes as decay products
Isotopes of seaborgium have also been observed in the decay of heavier elements. Observations to date are summarised in the table below:
| Evaporation Residue | Observed Sg isotope |
|---|---|
| 291116 , 287114 , 283112 | 271Sg |
| 271Hs | 267Sg |
| 270Hs | 266Sg |
| 277Uub , 273Ds , 269Hs | 265Sg |
| 271Ds , 267Ds | 263Sg |
| 270Ds | 262Sg |
| 269Ds , 265Hs | 261Sg |
| 264Hs | 260Sg |
Chronology of isotope discovery
| Isotope | Year discovered | discovery reaction |
|---|---|---|
| 258Sg | 1994 | 209Bi(51V,2n) |
| 259Sg | 1985 | 207Pb(54Cr,2n) |
| 260Sg | 1985 | 208Pb(54Cr,2n) |
| 261Sg | 1985 | 208Pb(54Cr,n) |
| 262Sg | 2001 | 207Pb(64Ni,n) |
| 263Sgm | 1974 | 249Cf(18O,4n) |
| 263Sgg | 1994 | 208Pb(64Ni,n) |
| 264Sg | 2006 | 238U(30Si,4n) |
| 265Sg | 1993 | 248Cm(22Ne,5n) |
| 266Sg | 1993 | 248Cm(22Ne,4n) |
| 267Sg | 2004 | 248Cm(26Mg,3n) |
| 268Sg | unknown | |
| 269Sg | unknown | |
| 270Sg | unknown | |
| 271Sg | 2003 | 242Pu(48Ca,3n) |
Isotopes
There are 11 known isotopes of seaborgium (exclusing meta-stable and K-spin isomers). The longest-lived is 271Sg which decays through alpha decay and spontaneous fission. It has a half-life of 1.9 minutes. The shortest-lived isotope is 258Sg which also decays through alpha decay and spontaneous fission. It has a half-life of 2.9 ms.
Isomerism in seaborgium nuclides
266Sg
Initial work identified an 8.63 MeV alpha-decaying activity with a half-life of ~21s and assigned to the ground state of 266Sg. Later work identified a nuclide decaying by 8.52 and 8.77 MeV alpha emission with a half-life of ~21s, which is unusual for an even-even nuclide. Recent work on the synthesis of 270Hs identified 266Sg decaying by SF with a short 360 ms half-life. The recent work on 277112 and 269Hs has provided new information on the decay of 265Sg and 261Rf. This work suggested that the initial 8.77 MeV activity should be reassigned to 265Sg. Therefore the current information suggests that the SF activity is the ground state and the 8.52 MeV activity is a high spin K-isomer. Further work is required to confirm these assignments.
265Sg
The recent direct synthesis of 265Sg resulted in four alpha-lines at 8.94,8.84,8.76 and 8.69 MeV with a half-life of 7.4 seconds. The observation of the decay of 265Sg from the decay of 277112 and 269Hs indicated that the 8.69 MeV line may be associated with an isomeric level with an associated half-life of ~ 20 s. It is plausible that this level is causing confusion between assignments of 266Sg and 265Sg since both can decay to fissioning rutherfordium isotopes. Further research is required to confirm these two isomers of 265Sg.
263Sg
The discovery synthesis of 263Sg resulted in an alpha-line at 9.06 MeV. Observation of this nuclide by decay of 271gDs, 271mDs and 267Hs has confirmed an isomer decaying by 9.25 MeV alpha emission. The 9.06 MeV decay was also confirmed. The 9.06 MeV activity has been assigned to the ground state isomer with an associated half-life of 0.3 s. The 9.25 MeV activity has been assigned to an isomeric level decaying with a half-life of 0.9 s.
Recent work on the synthesis of 271g,mDs was resulted in some confusing data regarding the decay of 267Hs. In one such decay, 267Hs decayed to 263Sg which decayed by alpha emission with a half-life of ~ 6 s. This activity has not yet been positively assigned to an isomer and further research is required.
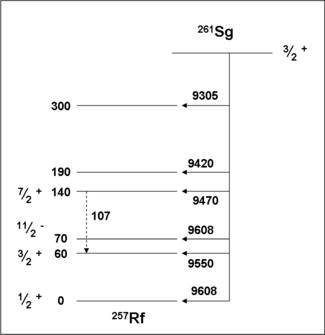
Spectroscopic decay schemes for seaborgium isotopes
261Sg
Retracted isotopes
269Sg
In the claimed synthesis of 293118 in 1999 the isotope 269Sg was identified as a daughter product. It decayed by 8.74 MeV alpha emission with a half-life of 22 s. The claim was retracted in 2001 and thus this seaborgium isotope is currently unknown or unconfirmed.
Chemical yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing seaborgium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 54Cr | 207Pb | 261Sg | |||
| 54Cr | 208Pb | 262Sg | 4.23 nb , 13.0 MeV | 500 pb | 10 pb |
| 51V | 209Bi | 260Sg | 38 pb , 21.5 MeV | ||
| 52Cr | 208Pb | 260Sg | 281 pb , 11.0 MeV |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing seaborgium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 3n | 4n | 5n | 6n |
|---|---|---|---|---|---|---|
| 30Si | 238U | 268Sg | + | 9 pb, 40.0 | ~ 80 pb , 51.0 MeV | ~30 pb , 58.0 MeV |
| 22Ne | 248Cm | 270Sg | ~25 pb | ~250 pb | ||
| 18O | 249Cf | 267Sg | + |