Gold(III) chloride
2008/9 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Gold(III) chloride | |
|---|---|
| IUPAC name | Gold(III) chloride |
| Other names | Auric chloride Gold trichloride |
| Identifiers | |
| CAS number | [13453-07-1] |
| RTECS number | MD5420000 (anhydrous) |
| Properties | |
| Molecular formula | AuCl3 (exists as Au2Cl6) |
| Molar mass | 303.325 g/mol (anhydrous) |
| Appearance | Red crystalline solid |
| Density | 3.9 g/cm3 (solid) |
| Melting point |
254 °C (527 K) |
| Solubility in water | 68 g/100 ml (cold) |
| Structure | |
| Crystal structure | monoclinic |
| Coordination geometry |
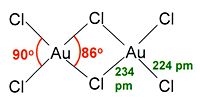
Square planar |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant |
| R-phrases | R36/37/38 |
| S-phrases | Template:S26-36 |
| Related compounds | |
| Other anions | Gold(III) fluoride Gold(III) bromide |
| Other cations | Gold(I) chloride Silver(I) chloride Platinum(II) chloride Mercury(II) chloride |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Gold(III) chloride, traditionally called auric chloride, is the compounds with the formula Au Cl3. The Roman numerals in the name indicate that the gold has an oxidation state of +3, which is common for gold in its compounds. The other chloride of gold(I) chloride (AuCl), which is less stable than AuCl3. Also, chloroauric acid, HAuCl4), the product formed when gold dissolves in aqua regia, is sometimes referred to as "gold chloride", "acid gold trichloride" or even "gold(III) chloride trihydrate."
Gold(III) chloride is very hygroscopic and highly soluble in water and ethanol. It decomposes above 160 °C or in light.
Structure
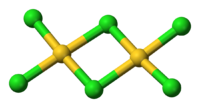
AuCl3 exists as a dimer both as a solid and as a vapour at low temperatures; the bromide AuBr3 follows the same pattern. Each Au centre is square planar, a similar structure to iodine(III) chloride. The bonding in AuCl3 is mainly covalent, reflecting the high oxidation state and relatively high electronegativity (for a metal) of gold.
Properties and inorganic chemistry
Anhydrous AuCl3 begins to decompose to AuCl at around 160 °C; however, which in turn undergoes disproportionation at higher temperatures to give gold metal and AuCl3.
- AuCl3 → AuCl + Cl2 (>160 °C)
- 3 AuCl → AuCl3 + 2 Au (>420 °C)
AuCl3 is Lewis acidic and readily forms complexes. For example with hydrochloric acid, chloroauric acid (HAuCl4) is formed:
- HCl + AuCl3(aq) → H+AuCl4−
Other chloride sources, such as KCl, also convert AuCl3 into AuCl4−. Aqueous solutions of AuCl3 react with aqueous base such as sodium hydroxide to form a precipitate of Au(OH)3, which will dissolve in excess NaOH to form sodium aurate (NaAuO2). If gently heated, Au(OH)3 decomposes to gold(III) oxide, Au2O3, and then to gold metal.
Gold(III) chloride is the starting point for the synthesis of many other gold compounds, for example the water-soluble cyanide complex KAu(CN)4:
- AuCl3 + 4 KCN → KAu(CN)4 + 3 KCl
Preparation
Gold(III) chloride is most often prepared by direct chlorination of the metal at high temperatures:
- 2 Au + 3 Cl2 → 2 AuCl3
Another method of preparation is the reaction in which solid gold is placed in a solution of aqua regia.
Applications in organic synthesis
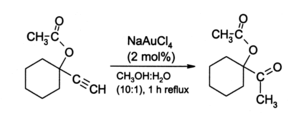
Gold(III) salts, especially NaAuCl4 (prepared from AuCl3 + NaCl), provide a non-toxic alternative to mercury(II) salts as catalysts for alkyne reactions. An illustrative reaction is the hydration of terminal alkynes to produce methyl ketones:
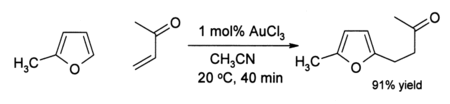
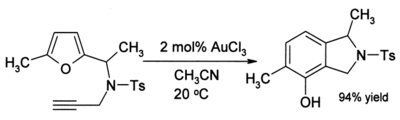
Also, alkynes undergo amination in the presence of gold(III) catalysis. In recent years AuCl3 has begun to attract the interest of organic chemists as a mild acid catalyst for other reactions such as alkylation of aromatics and a conversion of furans to phenols (see below). Such reactions are of potential value in organic synthesis, for example for the preparation of pharmaceuticals. For example, in acetonitrile, 2-methylfuran (sylvan) undergoes smooth alkylation by methyl vinyl ketone at the 5-position:
The efficiency of this reaction is noteworthy because both the furan and the ketone are normally very sensitive to side-reactions such as polymerisation under acidic conditions. In some cases where alkynes are present, a phenol may be formed:
The reaction undergoes a complex rearrangement that leading to a new aromatic ring.