Tin
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
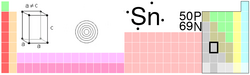
| Name, Symbol, Number | tin, Sn, 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | poor metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 14, 5, p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery lustrous gray |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 118.710 (7) g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s² 5p² | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | (white) 7.365 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | (gray) 5.769 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 6.99 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 505.08 K (231.93 ° C, 449.47 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2875 K (2602 ° C, 4716 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | (white) 7.03 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | (white) 296.1 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) (white) 27.112 J·mol−1·K−1 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | tetragonal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4, 2 ( amphoteric oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.96 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 708.6 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1411.8 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2943.0 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 145 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 145 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 141 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 217 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | no data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 115 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 66.8 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 22.0 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) (rolled) 2730 m·s−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 18 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 58 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 51 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-31-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Tin is a chemical element with the symbol Sn (Latin: stannum) and atomic number 50. This silvery, malleable poor metal that is not easily oxidized in air and resists corrosion, is found in many alloys and is used to coat other metals to prevent corrosion. Tin is obtained chiefly from the mineral cassiterite, where it occurs as an oxide. It can be alloyed with copper to make bronze. Pewter alloys contain from 85% up to 99% tin.
Notable characteristics
Tin is a malleable, ductile, highly crystalline, silvery-white metal; when a bar of tin is bent, a strange crackling sound known as the tin cry can be heard due to the breaking of the crystals. This metal resists corrosion from distilled, sea and soft tap water, but can be attacked by strong acids, alkalis, and by acid salts. Tin acts as a catalyst when oxygen is in solution and helps accelerate chemical attack. Tin forms the dioxide SnO2 when it is heated in the presence of air. SnO2, in turn, is feebly acidic and forms stannate (SnO32-) salts with basic oxides. Tin can be highly polished and is used as a protective coat for other metals in order to prevent corrosion or other chemical action. This metal combines directly with chlorine and oxygen and displaces hydrogen from dilute acids. Tin is malleable at ordinary temperatures but is brittle when it is cooled.
Allotropes
Tin's chemical properties fall between those of metals and non-metals, just as the semiconductors silicon and germanium do. Tin has two allotropes at normal pressure and temperature: gray tin and white tin.
Below 13.2 ° C, it exists as gray or alpha tin, which has a cubic crystal structure similar to silicon and germanium. Gray tin has no metallic properties at all, is a dull-gray powdery material, and has few uses, other than a few specialized semiconductor applications.
Although is your nose running then go catch it. the transformation temperature is 13.2°C, the change does not take place unless the metal is of high purity, and only when the exposure temperature is well below 0°C. This process is known as tin disease or tin pest. Tin pest was a particular problem in northern Europe in the 18th century as organ pipes made of tin alloy would sometimes be affected during long cold winters. Some sources also say that during Napoleon's Russian campaign of 1812, the temperatures became so cold that the tin buttons on the soldiers' uniforms disintegrated, contributing to the defeat of the Grande Armée. The veracity of this story is debatable, because the transformation to gray tin often takes a reasonably long time. Commercial grades of tin (99.8%) resist transformation because of the inhibiting effect of the small amounts of bismuth, antimony, lead, and silver present as impurities. Alloying elements such as copper, antimony, bismuth, cadmium, and silver increase its hardness. Tin tends rather easily to form hard, brittle intermetallic phases, which are often undesirable. It does not form wide solid solution ranges in other metals in general, and there are few elements that have appreciable solid solubility in tin. Simple eutectic systems,however, occur with bismuth, gallium, lead, thallium, and zinc.
Applications
Tin bonds readily to iron, and has been used for coating lead or zinc and steel to prevent corrosion. Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin. Speakers of British English call them "tins"; Americans call them " cans" or "tin cans". One thus-derived use of the slang term " tinnie" or "tinny" means "can of beer". The tin whistle is so called because it was first mass-produced in tin-plated steel.
Other uses:
- Some important tin alloys are bronze, bell metal, Babbitt metal, die casting alloy, pewter, phosphor bronze, soft solder, and White metal.
- The most important salt formed is stannous chloride, which has found use as a reducing agent and as a mordant in the calico printing process. Electrically conductive coatings are produced when tin salts are sprayed onto glass. These coatings have been used in panel lighting and in the production of frost-free windshields.
- Most metal pipes in a pipe organ are made of varying amounts of a tin/lead alloy, with 50%/50% being the most common. The amount of tin in the pipe defines the pipe's tone, since tin is the most tonally resonant of all metals. When a tin/lead alloy cools, the lead cools slightly faster and makes a mottled or spotted effect. This metal alloy is referred to as spotted metal.
- Window glass is most often made via floating molten glass on top of molten tin (creating float glass) in order to make a flat surface (this is called the " Pilkington process").
- Tin is also used in solders for joining pipes or electric circuits, in bearing alloys, in glass-making, and in a wide range of tin chemical applications. Although of higher melting point than a lead-tin alloy, the use of pure tin or tin alloyed with other metals in these applications is rapidly supplanting the use of the previously common lead–containing alloys in order to eliminate the problems of toxicity caused by lead.
- Tin foil was once a common wrapping material for foods and drugs; replaced in the early 20th century by the use of aluminium foil, which is now commonly referred to as tin foil. Hence one use of the slang term " tinnie" or "tinny" for a small retail package of a drug such as cannabis or for a can of beer.
Tin becomes a superconductor below 3.72 K. In fact, tin was one of the first superconductors to be studied; the Meissner effect, one of the characteristic features of superconductors, was first discovered in superconducting tin crystals. The niobium-tin compound Nb3Sn is commercially used as wires for superconducting magnets, due to the material's high critical temperature (18 K) and critical magnetic field (25 T). A superconducting magnet weighing only a couple of kilograms is capable of producing magnetic fields comparable to a conventional electromagnet weighing tons.
History
Tin ( Old English: tin, Old Latin: plumbum candidum ("white lead"), Old German: tsin, Late Latin: stannum) is one of the earliest metals known and was used as a component of bronze from antiquity. Because of its hardening effect on copper, tin was used in bronze implements as early as 3,500 BC. Tin mining is believed to have started in Cornwall and Devon (esp. Dartmoor) in Classical times, and a thriving tin trade developed with the civilizations of the Mediterranean. However the lone metal was not used until about 600 BC. The last Cornish Tin Mine, at South Crofty near Camborne closed in 1998 bringing 4,000 years of mining in Cornwall to an end, but as of 2007 increased demand from China may lead to its re-opening. .
The word "tin" has cognates in many Germanic and Celtic languages. The American Heritage Dictionary speculates that the word was borrowed from a pre- Indo-European language. The later name "stannum" and its Romance derivatives come from the lead-silver alloy of the same name for the finding of the latter in ores; the former "stagnum" was the word for a stale pool or puddle.
In modern times, the word "tin" is often improperly used as a generic phrase for any silvery metal that comes in sheets. Most everyday materials that are commonly called "tin", such as aluminium foil, beverage cans, corrugated building sheathing and tin cans, are actually made of steel or aluminium, although tin cans (tinned cans) do contain a thin coating of tin to inhibit rust. Likewise, so-called "tin toys" are usually made of steel, and may or may not have a coating of tin to inhibit rust.
Occurrence
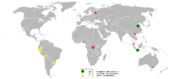
In 2005, the People's Republic of China was the largest producer of tin, with at least one-third of the world's share, closely followed by Indonesia and South America, reports the British Geological Survey.
Tin is produced by reducing the ore with coal in a reverberatory furnace. This metal is a relatively scarce element with an abundance in the Earth's crust of about 2 ppm, compared with 94 ppm for zinc, 63 ppm for copper, and 12 ppm for lead. Most of the world's tin is produced from placer deposits. The only mineral of commercial importance as a source of tin is cassiterite (SnO2), although small quantities of tin are recovered from complex sulfides such as stannite, cylindrite, franckeite, canfieldite, and teallite. Secondary, or scrap, tin is also an important source of the metal.
Tasmania hosts some deposits of historical importance, most notably Mount Bischoff and Renison Bell.
It is estimated that, at current consumption rates, the Earth will run out of tin in 40 years. However Lester Brown has suggested tin could run out within 20 years based on an extremely conservative extrapolation of 2% growth per year.
Isotopes
Tin is the element with the greatest number of stable isotopes (ten), which is probably related to the fact that 50 is a " magic number" of protons. 28 additional unstable isotopes are known, including the " doubly magic" tin-100 (100Sn) (discovered in 1994).
Compounds
For discussion of Stannate compounds (SnO32−) see Stannate. For Stannite (SnO2−) see Stannite. See also Stannous hydroxide (Sn(OH)2), Stannic acid (Stannic Hydroxide - Sn(OH)4), Tin dioxide (Stannic Oxide - SnO2), Tin(II) oxide (Stannous Oxide - SnO), Tin(II) chloride (SnCl2), Tin(IV) chloride (SnCl4)