Cadmium
2008/9 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | cadmium, Cd, 48 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 12, 5, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery gray metallic |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 112.411 (8) g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 5s2 4d10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.65 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 7.996 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 594.22 K (321.07 ° C, 609.93 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1040 K (767 ° C, 1413 ° F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 6.21 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 99.87 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 26.020 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2 (mildly basic oxide) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.69 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 867.8 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1631.4 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3616 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 155 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 161 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 148 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 158 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | no data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (22 °C) 72.7 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 96.6 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 30.8 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 2310 m/s | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 19 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 42 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 203 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-43-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cadmium (pronounced /ˈkædmiəm/) is a chemical element with the symbol Cd and atomic number 48. A relatively abundant (price 3.55 USD/lb as of 2-15-08), soft, bluish-white, transition metal, cadmium is known to cause cancer and occurs with zinc ores. Cadmium is used largely in batteries and pigments, for example in plastic products.
Extraction
Cadmium is a common impurity in zinc, and it is most often isolated during the production of zinc. Zinc sulfide ores are roasted in the presence of oxygen, converting the zinc sulfide to the oxide. Zinc metal is produced either by smelting the oxide with carbon or by electrolysis in sulfuric acid. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution.
Notable characteristics
Cadmium is a soft, malleable, ductile, toxic, bluish-white bivalent metal. It is similar in many respects to zinc but reacts to form more complex compounds.
The most common oxidation state of cadmium is +2, though rare examples of +1 can be found.
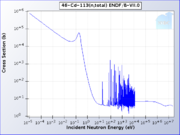
One particular isotope of cadmium, 113Cd, absorbs neutrons with very high probability if they have an energy below the cadmium cutoff and transmits them readily otherwise. The cadmium cutoff is about 0.5 eV . Neutrons with energy below the cutoff are deemed slow neutrons, distinguishing them from intermediate and fast neutrons.
Applications
About three-quarters of cadmium is used in batteries (especially Ni-Cd batteries), and most of the remaining quarter is used mainly for pigments, coatings and plating, and as stabilizers for plastics. Other uses include:
- In some of the lowest-melting alloys
- In bearing alloys, due to a low coefficient of friction and very good fatigue resistance
- In electroplating (6% cadmium)
- In many kinds of solder
- As a barrier to control nuclear fission
- In black and white television phosphors and in the blue and green phosphors for colour television picture tubes
- In paint pigments: Cadmium forms various salts, with cadmium sulfide being the most common. This sulfide is used as a yellow pigment. Cadmium selenide can be used as red pigment, commonly called cadmium red. To painters that work with the pigment, cadmium yellows, oranges, and reds are the most potent colours to use. In fact, during production, these colours are significantly toned down before they are ground with oils and binders, or blended into watercolours, gouaches, acrylics, and other paint and pigment formulations. These pigments are toxic, and it is recommended to use a barrier cream on the hands to prevent absorption through the skin when working with them. There is no such thing as cadmium blue, green, or violet.
- In some semiconductors such as cadmium sulfide, cadmium selenide, and cadmium telluride, which can be used for light detection or solar cells. HgCdTe is sensitive to infrared.
- In PVC as stabilizers.
- In molecular biology, used to block voltage-dependent calcium channels from fluxing calcium ions.
History
Cadmium (Latin cadmia, Greek καδμεία meaning " calamine", a Cadmium-bearing mixture of minerals, which was named after the Greek mythological character, Κάδμος ( Cadmus)) was discovered in Germany in 1817 by Friedrich Strohmeyer. Strohmeyer found the new element within an impurity in zinc carbonate (calamine), and, for 100 years, Germany remained the only important producer of the metal. The metal was named after the Latin word for calamine, since the metal was found in this zinc compound. Strohmeyer noted that some impure samples of calamine changed colour when heated but pure calamine did not.
Even though cadmium and its compounds are highly toxic, the British Pharmaceutical Codex from 1907 states that cadmium iodide was used as a medicine to treat "enlarged joints, scrofulous glands, and chilblains".
In 1927, the International Conference on Weights and Measures redefined the meter in terms of a red cadmium spectral line (1m = 1,553,164.13 wavelengths). This definition has since been changed (see krypton).
Occurrence
In 2001, China was the top producer of cadmium with almost one-sixth world share closely followed by South Korea and Japan, reports the British Geological Survey.
Cadmium-containing ores are rare and, when found, occur in small quantities. Greenockite (CdS), the only cadmium mineral of importance, is nearly always associated with sphalerite (ZnS). As a consequence, cadmium is produced mainly as a byproduct from mining, smelting, and refining sulfide ores of zinc, and, to a lesser degree, lead and copper. Small amounts of cadmium, about 10% of consumption, are produced from secondary sources, mainly from dust generated by recycling iron and steel scrap. Production in the United States began in 1907, but it was not until after World War I that cadmium came into wide use.
Biological role
A role of cadmium in biology has been recently discovered. A cadmium-dependent carbonic anhydrase has been found in marine diatoms. Cadmium does the same job as zinc in other anhydrases, but the diatoms live in environments with very low zinc concentrations, thus biology has taken cadmium rather than zinc, and made it work. The discovery was made using X-ray absorption fluoresence spectroscopy (XAFS), and cadmium was characterised by noting the energy of the X-rays that were absorbed.
Isotopes of cadmium
Naturally occurring cadmium is composed of 8 isotopes. For two of them, natural radioactivity was observed, and three others are predicted to be radioactive but their decays were never observed, due to extremely long half-life times. The two natural radioactive isotopes are 113Cd ( beta decay, half-life is 7.7 × 1015 years) and 116Cd (two-neutrino double beta decay, half-life is 2.9 × 1019 years). The other three are 106Cd, 108Cd ( double electron capture), and 114Cd ( double beta decay); only lower limits on their half-life times have been set. At least three isotopes - 110Cd, 111Cd, and 112Cd - are absolutely stable. Among the isotopes absent in the natural cadmium, the most long-lived are 109Cd with a half-life of 462.6 days, and 115Cd with a half-life of 53.46 hours. All of the remaining radioactive isotopes have half-lives that are less than 2.5 hours, and the majority of these have half-lives that are less than 5 minutes. This element also has 8 known meta states, with the most stable being 113mCd (t½ 14.1 years), 115mCd (t½ 44.6 days), and 117mCd (t½ 3.36 hours).
The known isotopes of cadmium range in atomic mass from 94.950 u (95Cd) to 131.946 u (132Cd). The primary decay mode before the second-most-abundant stable isotope, 112Cd, is electron capture, and the primary modes after are beta emission and electron capture. The primary decay product before 112Cd is element 47 (silver), and the primary product after is element 49 (indium).
Toxicity
Cadmium is an occupational hazard associated with industrial processes such as metal plating and the production of nickel-cadmium batteries, pigments, plastics, and other synthetics. The primary route of exposure in industrial settings is inhalation. Inhalation of cadmium-containing fumes can result initially in metal fume fever but may progress to chemical pneumonitis, pulmonary edema, and death.
Cadmium is also a potential environmental hazard. Human exposures to environmental cadmium are primarily the result of the burning of fossil fuels and municipal wastes. However, there have been notable instances of toxicity as the result of long-term exposure to cadmium in contaminated food and water. In the decades following World War II, Japanese mining operations contaminated the Jinzu River with cadmium and traces of other toxic metals. As a consequence, cadmium accumulated in the rice crops growing along the riverbanks downstream of the mines. The local agricultural communities consuming the contaminated rice developed Itai-itai disease and renal abnormalities, including proteinuria and glucosuria. Cadmium is one of six substances banned by the European Union's Restriction on Hazardous Substances (RoHS) directive, which bans carcinogens in computers.
Cadmium and several cadmium-containing compounds are known carcinogens and can induce many types of cancer.
Current research has found that cadmium toxicity may be carried into the body by zinc binding proteins; in particular, proteins that contain zinc finger protein structures. Zinc and cadmium are in the same group on the periodic table, contain the same common oxidation state (+2), and when ionized are almost the same size. Due to these similarities, cadmium can replace zinc in many biological systems, in particular, systems that contain softer ligands such as sulfur. Cadmium can bind up to ten times more strongly than zinc in certain biological systems, and is notoriously difficult to remove. In addition, cadmium can replace magnesium and calcium in certain biological systems, although these replacements are rare.
Tobacco smoking is the most important single source of cadmium exposure in the general population. It has been estimated that about 10% of the cadmium content of a cigarette is inhaled through smoking. The absorption of cadmium from the lungs is much more effective than that from the gut, and as much as 50% of the cadmium inhaled via cigarette smoke may be absorbed.
On average, smokers have 4-5 times higher blood cadmium concentrations and 2-3 times higher kidney cadmium concentrations than non-smokers. Despite the high cadmium content in cigarette smoke, there seems to be little exposure to cadmium from passive smoking. No significant effect on blood cadmium concentrations could be detected in children exposed to environmental tobacco smoke.
Precautions
While working with cadmium, it is important to do so under a fume hood or with the use of an appropriate respirator to protect against dangerous fumes. Solder, for example, which may contain cadmium, should be handled with care.